Abstract
Cordyceps bassiana has long been used as an oriental medicine and reported to possess diverse biological activities. The fruiting bodies of Cordyceps bassiana was extracted with ethanol and then further fractionated with n-hexane, ethyl acetate, n-butanol and water. The butanol fraction from Cordyceps bassiana (CBBF) exhibited the most effective in anti-inflammatory activity in RAW 264.7 macrophages and the roles of CBBF on the anti-inflammation cascade in LPS-stimulated RAW 264.7 cells were studied. To investigate the mechanism by which CBBF inhibits NO, iNOS and COX-2, the activation of IκB and MAPKs in LPS-activated macrophage were examined. Our present results demonstrated that CBBF inhibits NO production and iNOS expression in LPS-stimulated RAW 264.7 macrophage cells, and these effects were mediated through the inhibition of IκB-α, JNK and p38 phosphorylation. Also, CBBF suppressed activation of MAPKs including p38 and SAPK/JNK. Furthermore, CBBF significantly suppressed LPS-induced intracellular ROS generation. Its inhibition on iNOS expression, together with its antioxidant activity, may support its anti-inflammatory activity. Thus Cordyceps bassiana can be used as a useful medicinal food or drug for further studies.
Go to : 
References
(1). Zhou X.., Gong Z.., Su Y.., Lin J.., Tang K. J.Pharm. Pharmacol. 2009. 61:279–291.
(2). Sung G. H.., Hywel-Jones N. L.., Sung J. M.., Luangsa-Ard J. J.., Shrestha B.., Spatafora J. W.Stud. Mycol. 2007. 57:5–59.
(3). Priest F. G.., Goodfellow M.Applied Microbial Systematics (9eds.); Kluwer Academic Publishers: Dordrecht. P,. 2000. 203–230.
(4). Schaeffenberg B. Z.Pflanzenkrankh. Pflanzensch. 1955. 62:544–549.
(5). Li Z.., Li C.., Huang B.., Fan M.Chinese Science Bulletin. 2001. 46:751–753.
(6). Kim K. M.., Kwon Y. G.., Chung H. T.., Yun Y. G.., Pae H. O.., Han J. A.., Ha K. S.., Kim T. W.., Kim Y. M.Toxicol. Appl. Pharmacol. 2003. 190:1–8.
(7). Park Y. M.., Won J. H.., Kim Y. H.., Choi J. W.., Park H. J.., Lee K. T. J.Ethnopharmacol. 2005. 101:120–128.
(8). Kim B. C.., Choi J. W.., Hong H. Y.., Lee S. A.., Hong S.., Park E. H.., Kim S. J.., Lim C. J. J.Ethnopharmacol. 2006. 106:364–371.
(9). Paterson R. R.Phytochemistry. 2008. 69:1469–1495.
(10). Wadsworth T. L.., Koop D. R.Biochem. Pharmacol. 1999. 57:941–949.
(12). D'Acquisto F.., May M. J.., Ghosh S.Mol. Interv. 2002. 2:22–35.
(13). Moynagh P. N. J.Cell Sci. 2005. 118:4589–4592.
(14). Giri S.., Rattan R.., Singh A. K.., Singh I. J.Immunol. 2004. 173:5196–5208.
(15). Islam S.., Hassan F.., Mu M. M.., Ito H.., Koide N.., Mori I.., Yoshida T.., Yokochi T.Microbiol. Immunol. 2004. 48:729–736.
(16). Chan E. D.., Riches D. W.Am. J. Physiol. Cell Physiol. 2001. 280:C441–C450.
(17). Hommes D. W.., Peppelenbosch M. P.., van Deventer S. J.Gut. 2003. 52:144–151.
(18). Kim S. H.., Johnson V. J.., Shin T. Y.., Sharma R. P.Exp. Biol. Med(Maywood). 2004. 229:203–213.
(19). Bai S. K.., Lee S. J.., Na H. J.., Ha K. S.., Han J. A.., Lee H.., Kwon Y. G.., Chung C. K.., Kim Y. M.Exp. Mol. Med. 2005. 37:323–334.
(20). Kim J. H.., Kim D. H.., Baek S. H.., Lee H. J.., Kim M. R.., Kwon H. J.., Lee C. H.Biochem. Pharmacol. 2006. 71:1198–1205.
(21). Torres M.., Forman H. J.Biofactors. 2003. 17:287–296.
(22). Suh S. J.., Chung T. W.., Son M. J.., Kim S. H.., Moon T. C.., Son K. H.., Kim H. P.., Chang H. W.., Kim C. H.Arch. Biochem. Biophys. 2006. 447:136–146.
(23). Suh W.., Nam G.., Yang W. S.., Sung G. H.., Shim S. H.., Cho J. Y.Biomol. Ther. 2017. 25:165–170.
Go to : 
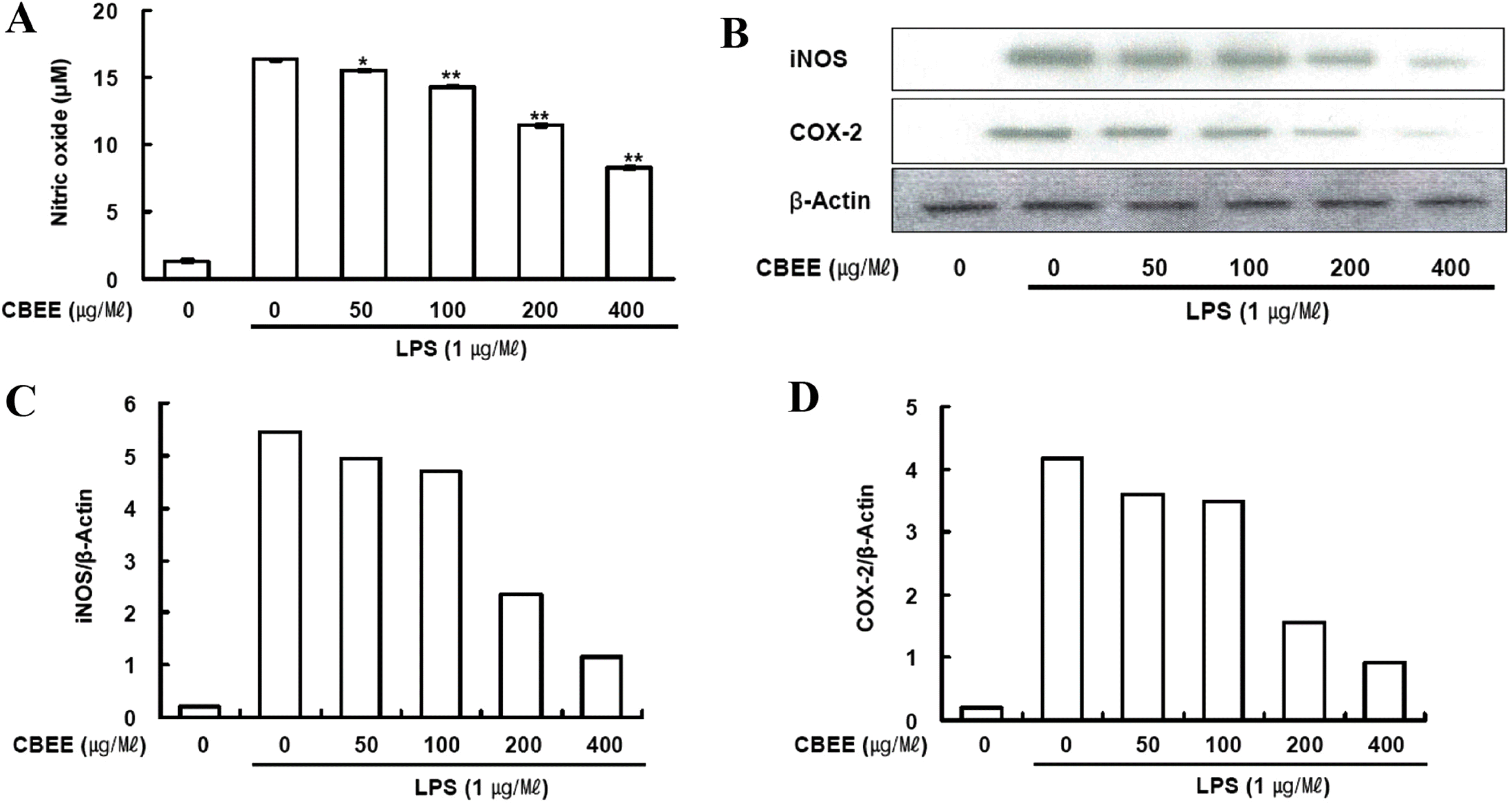 | Fig. 1.Effects of CBEE on NO production, iNOS and COX-2 protein expression in LPS-induced macrophages: (A) RAW 264.7 cells (4 × 106 cells/well) were incubated for 16 h with 1 μg/ml LPS in the presence or absence of CBEE. RAW 264.7 cells were pretreated with the indicated concentrations of CBEE for 30 min before incubation with LPS for 16 h. The culture supernatants were subsequently isolated and analyzed for nitrite levels. Data are expressed as mean ± S.D.(n = 3). Asterisks indicate a significant difference from LPS alone (∗ P < 0.05, ∗∗ P < 0.01 vs. LPS alone). (B) The cells were lysed, and the lysates were analyzed by immunoblotting used anti-iNOS and anti-COX-2. The blot was stripped from the bound antibody and reprobed with anti-β-actin to confirm equal loading. (C∼D) The intensity of the bands was scanned and quantified by Scion Image software. |
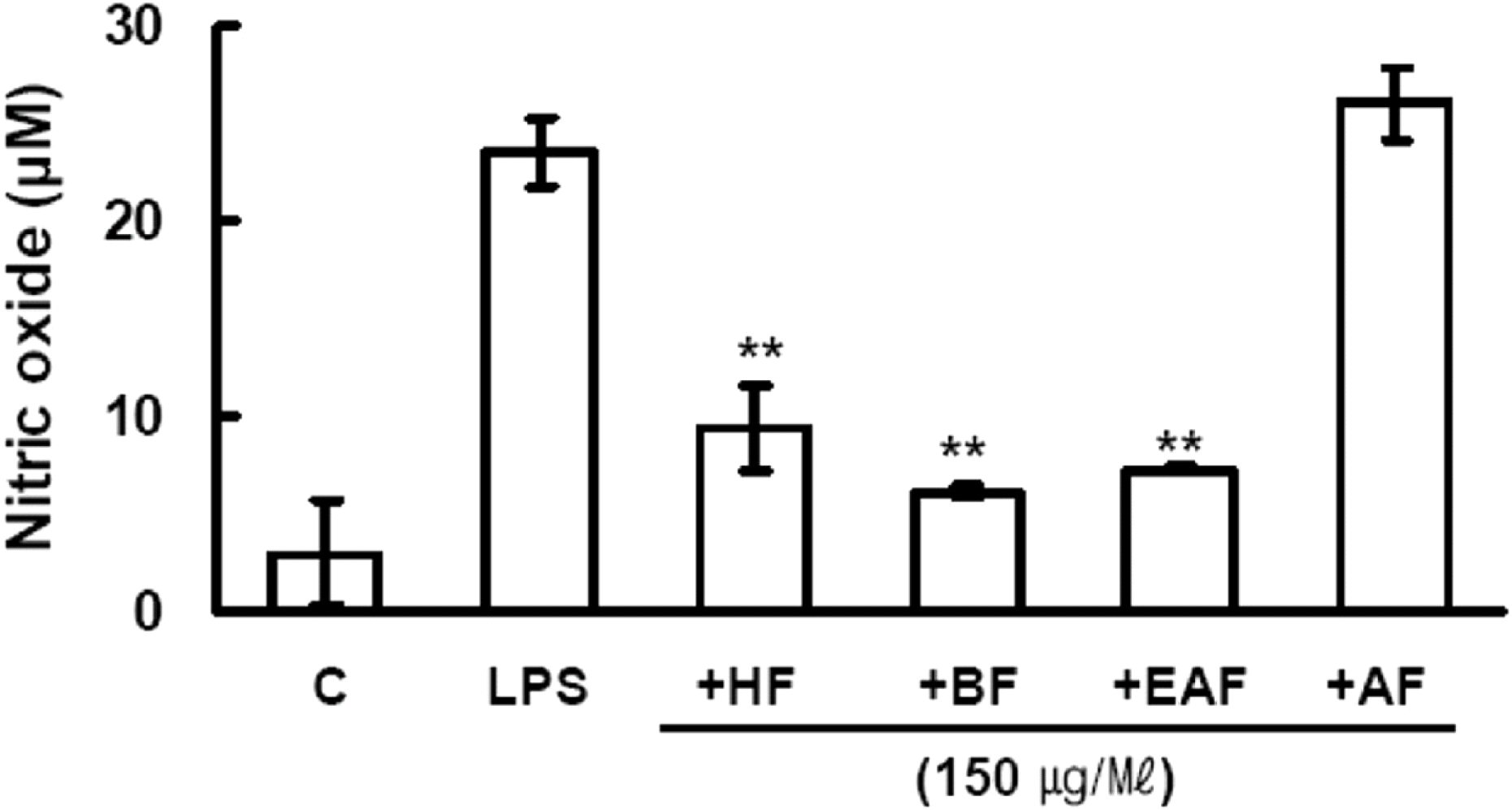 | Fig. 2.Effect of Cordyceps bassiana solvent fractions on NO production in LPS-induced macrophages. RAW 264.7 cells (4 × 106 cells/well) were incubated for 16 h with 1 μg/ml LPS in the presence or absence of CBEE. RAW 264.7 cells were pretreated with 150 μg/ml of various solvent fractions for 30 min before incubation with LPS for 16 h. HF, n-hexane fraction; BF, n-butanol fraction; EAF, ethyl acetate fraction; AF, aqueous fraction. The culture supernatants were subsequently isolated and analyzed for nitrite levels. Data are expressed as mean ± S.D. (n = 3). Asterisks indicate a significant difference from LPS alone (∗ P < 0.05, ∗∗ P < 0.01 vs. LPS alone). |
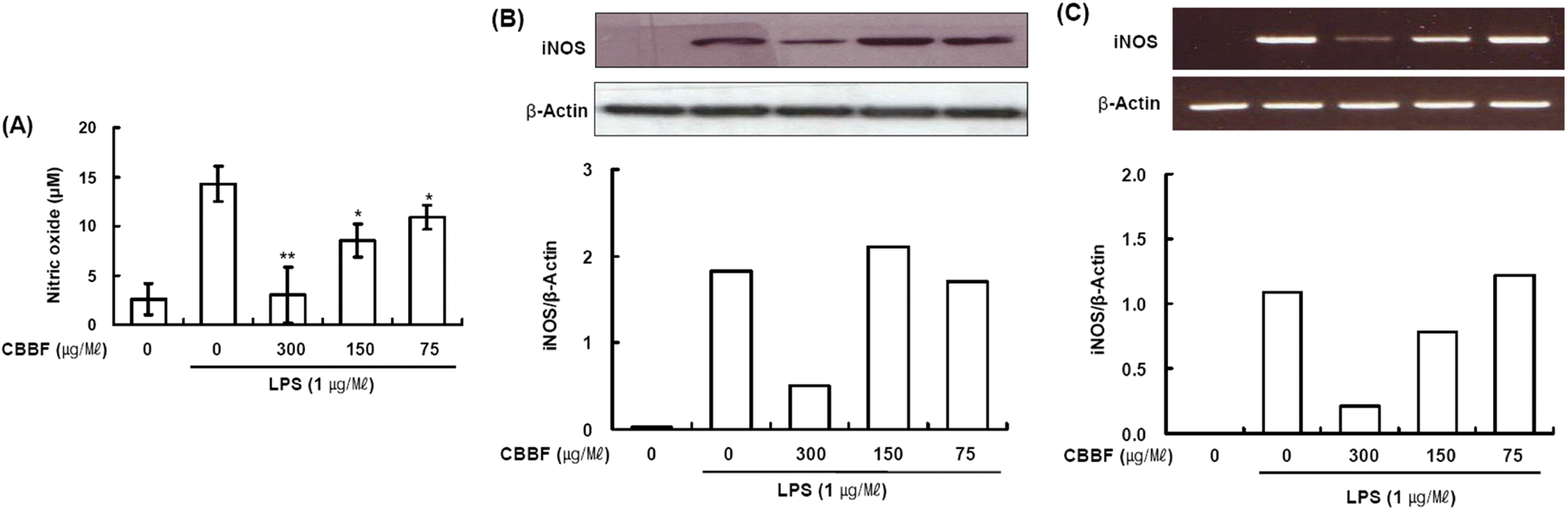 | Fig. 3.Effects of CBBF on NO production, iNOS protein and mRNA expression in LPS-induced macrophages. RAW 264.7 cells (4 × 106 cells/well) were incubated with 1 μg/ml LPS in the presence or absence of CBBF. (A) RAW 264.7 cells were pretreated with indicated concentrations of CBBF for 30 min before incubation with LPS for 16 h. The culture supernatants were subsequently isolated and analyzed for nitrite levels. Data are expressed as mean ± S.D. (n = 3). Asterisks indicate a significant difference from LPS alone (∗P < 0.05, ∗∗P < 0.01 vs. LPS alone). (B) The cells were lysed, and the lysates were analyzed by immunoblotting used anti-iNOS. The blot was stripped from the bound antibody and reprobed with anti-β-actin to confirm equal loading. (C) After 8 h stimulation, total RNAs were obtained from RAW 264.7 cells using a Trizol reagent kit. The mRNA levels of iNOS was determined by RT-PCR analysis. Actin was used as an internal control. |
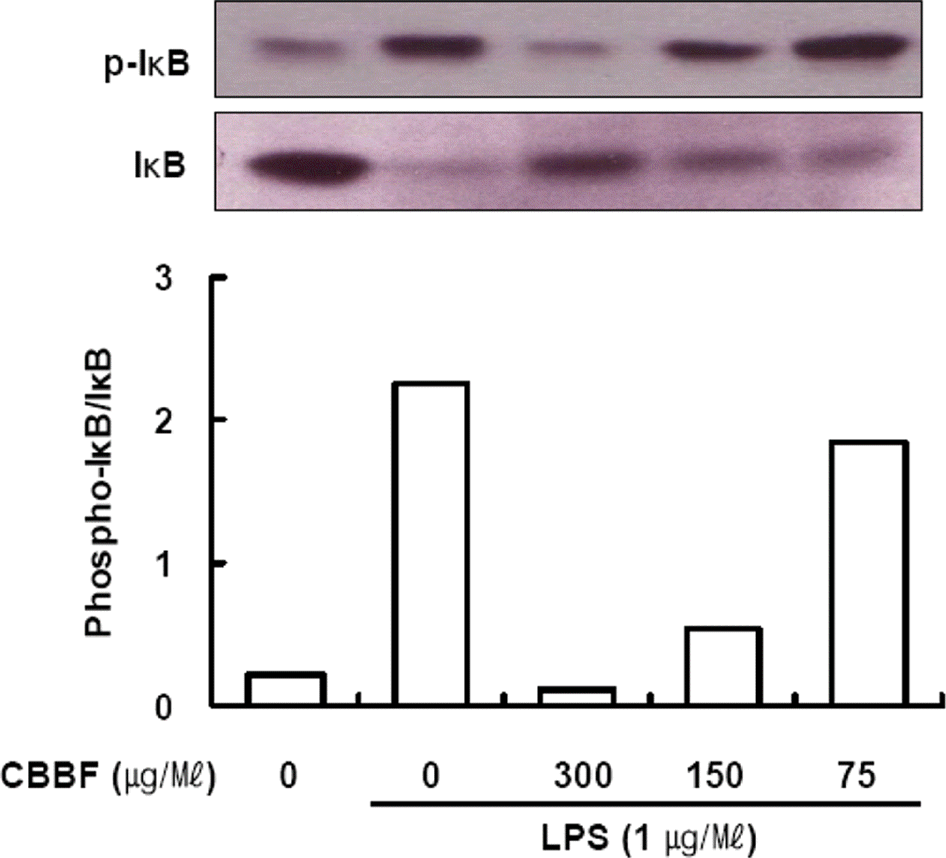 | Fig. 4.Effect of CBBF on LPS-induced phosphorylation of IκB-α. Raw 264 Macrophages were incubated with LPS (1 μg/ml) without or with different concentration of CBBF for 2 h. Cytosolic extracts of the cells were subjected to Western blot analysis with anti-phospho-IκBα (Ser-32/36) antibody and anti-IκBα. |
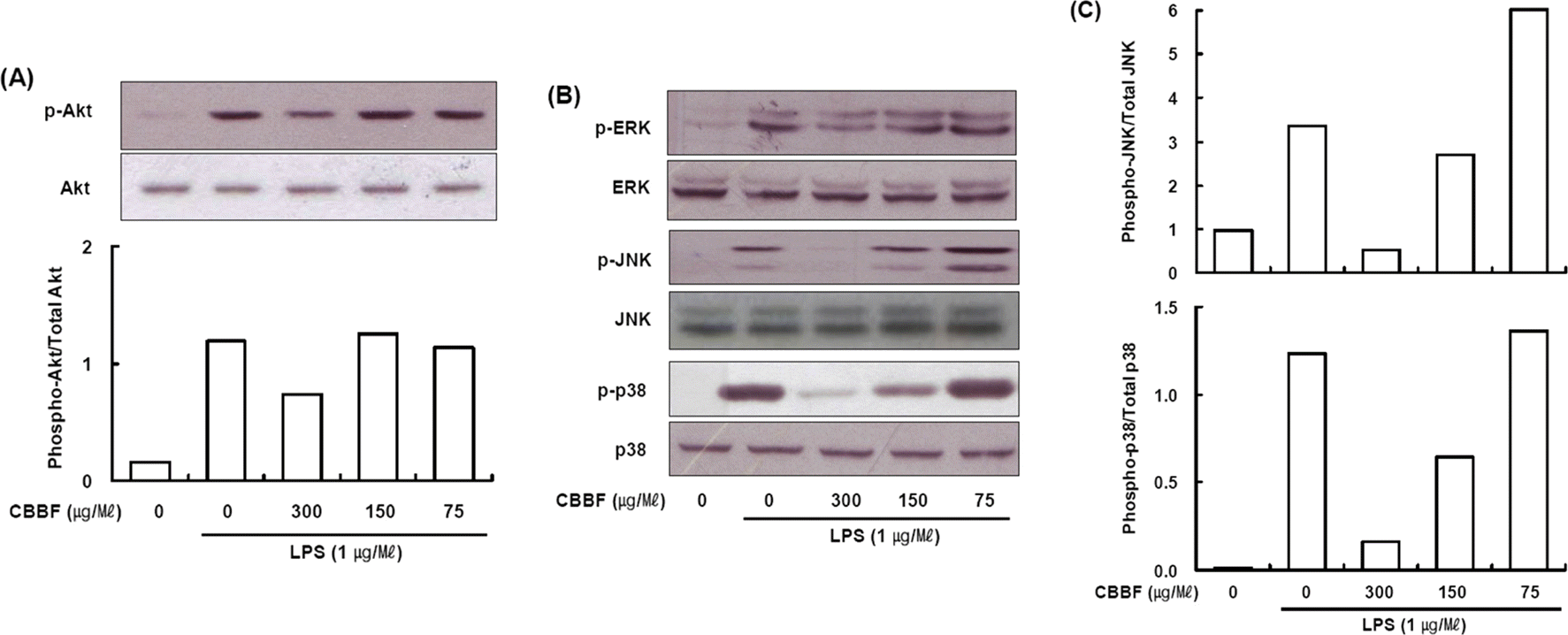 | Fig. 5.Effects of CBBF on Akt and MAPKs activation in RAW 264.7 macrophage cells: (4 × 106 cells/well) were treated with or without LPS (1 μg/ml), or with LPS plus different concentrations of CBBF. Control values were obtained in case of LPS and CBBF. CBBF was pretreated for 30 min and then treated with LPS for 30 min. Western blot analysis was performed using a specific antibody raised against Akt (A) and MAPKs (B). (C) The intensity of the bands was scanned and quantified. |
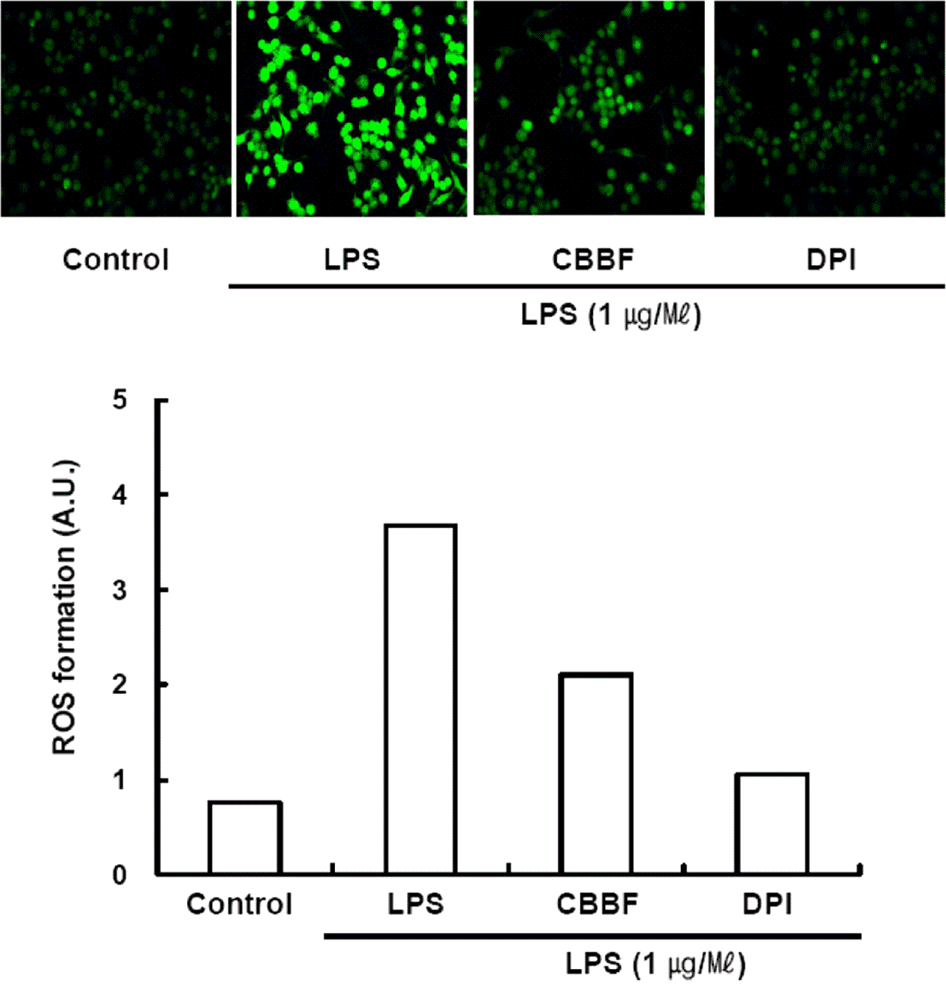 | Fig. 6.Effect of CBBF on LPS-activated ROS production. RAW 264.7 cells were treated with LPS in the presence or absence of CBBF (150 μg/ml). After 30 min incubation, cells were incubated with DCFH2-DA (5 μM) for an additional 30 min. Cells were washed twice with PBS, and the intracellular levels of ROS were analyzed by confocal microscope. DCF fluorescence intensities were determined from the same numbers of cells in randomly selected area. DPI, NADPH oxidase inhibitor, 10 μM. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download