Abstract
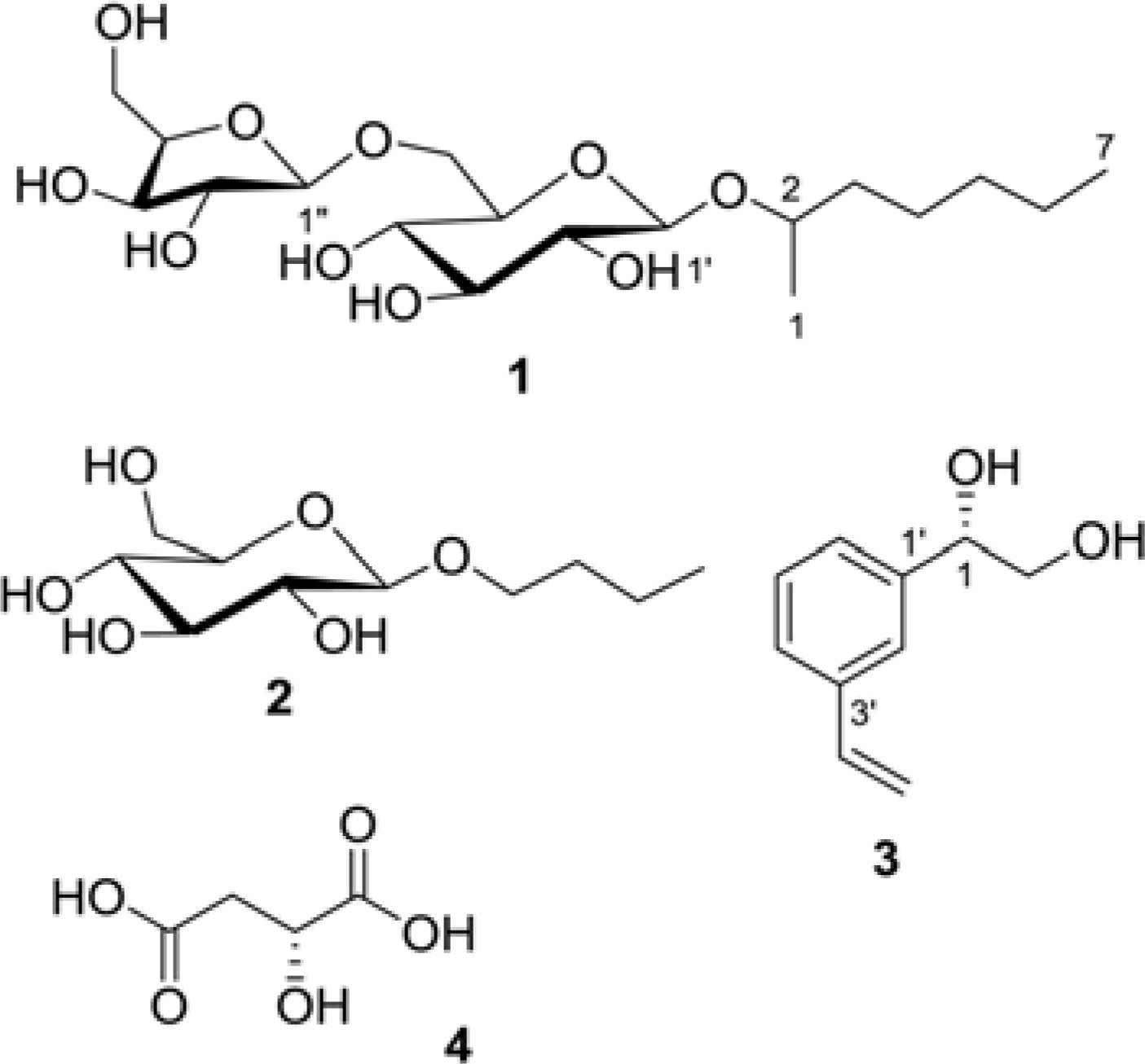
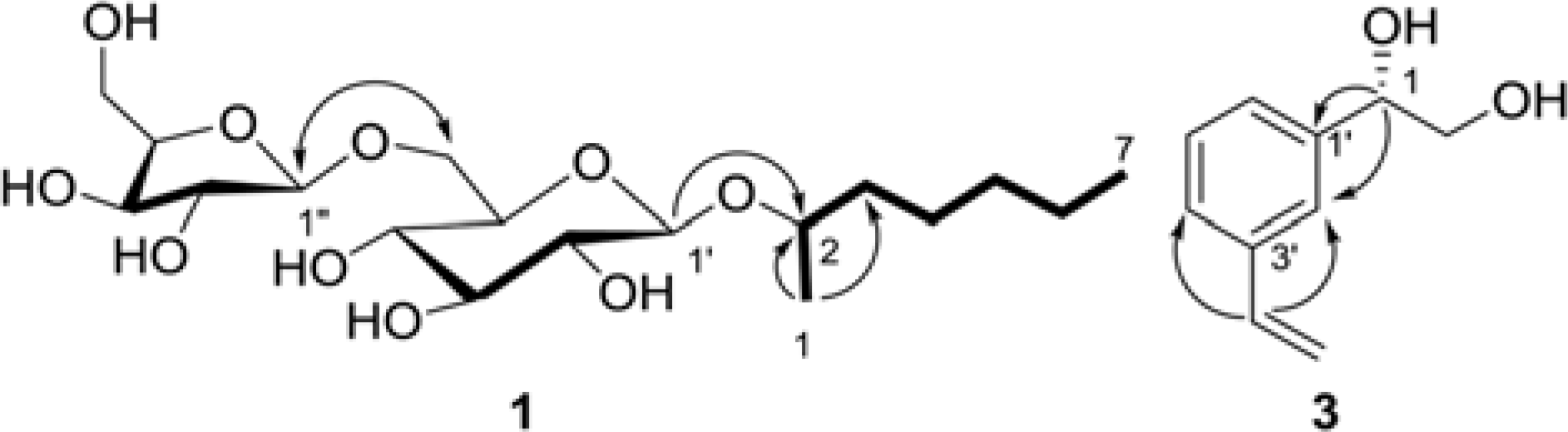
In a continuing study of the fermented noni (Morinda citrifolia) juice exudates, five compounds, heptanyl 2-O-β-D-xylofuranosyl-(1 → 6)-β-D-glucopyranoside (1), n-butyl β-D-glucopyranoside (2), (1S)-(3-ethenyl-phenyl)-1,2-ethanediol (3), (2S)-2-hydroxybutanedioic acid (4), and daucosterol (5) were isolated from the buthanol partition of the extract. The structures of the isolates were identified by 1D and 2D NMR, and MS experiments, as well as by comparison of their data with the published values. Among the isolates, compounds 1 – 3 were isolated for the first time from the plant species. The isolated compounds were evaluated for their cancer chemopreventive potential based on their ability to inhibit nitric oxide (NO) production and tumor necrosis factor alpha (TNF-α)-induced NF-κB activity, and quinonone reductase-1 (QR1)-inducing effect.
Go to : 
References
(1). Wang M.Y.., West B. J.., Jensen C. J.., Nowicki D.., Su C.., Palu A. K.., Anderson G.Acta Pharmacol. Sin. 2002. 23:1127–1141.
(2). Hirazumi A.., Furusawa E.., Chou S. C.., Hokama Y.Proc. West Pharmacol. Soc. 1996. 39:7–9.
(3). Palu A. K.., Kim A. H.., West B. J.., Deng S.., Jensen J.., White L. J.Ethnopharmacol. 2008. 115:502–506.
(4). Yang J.., Paulino R.., Janke-Stedronsky S.., Abawi F.Food Chem. 2007. 102:302–308.
(5). Jain R. K.., Srivastava S. D.Proc. Nat. Acad. Sci. India. 1992. 62A:11–13.
(6). Deng Y.., Chin Y. W.., Chai H.., Keller W. J.., Kinghorn A. D. J.Nat. Prod. 2007. 70:2049–2052.
(7). Srivastava M.., Singh J.Int. J. Pharmacog. 1993. 31:182–184.
(8). Liu G.., Bode A.., Ma W. Y.., Sang S.., Ho C. T.., Dong Z.Cancer Res. 2001. 61:5749–5756.
(9). Su B. N.., Pawlus A. D.., Jung H. A.., Keller W. J.., McLaughlin J. L.., Kinghorn A. D. J.Nat. Prod. 2005. 68:592–595.
(10). Kanchanapoom T.., Kasai R.., Yamasaki K.Phytochemistry. 2002. 59:551–556.
(11). Sang S.., Cheng X.., Zhu N.., Wang M.., Jhoo J. W.., Stark R. E.., Badmaev V.., Ghai G.., Rosen R. T.., Ho C.T. J.Nat. Prod. 2001. 64:799–800.
(12). Youn U. J.., Park E. J.., Kondratyuk T. P.., Sang-Ngern M.., Wall M. M.., Wei Y.., Pezzuto J. M.., Chang L. C. J.Nat. Prod. 2016. 79:1508–1513.
(13). Kondratyuk T. P.., Park E. J.., Yu R.., van Breemen R.B.., Asolkar R. N.., Murphy B. T.., Fenical W.., Pezzuto J. M.Mar. Drugs. 2012. 1:451–464.
(14). Park E.J.., Kondratyuk T.P.; Morrell, A.; Kiselev, E.; Conda-Sheridan, M.; Cushman, M.; Ahn, S.; Choi, Y.; White, J.J.; van Breemen, R.B.; Pezzuto, J.M. Cancer Prev. Res (Phila). 2011. 4:592–607.
(15). Song L. L.., Kosmeder II J. W.., Lee S. K.., Gerhäuser C.., Lantvit D.., Moon R. C.., Moriarty R. M.., Pezzuto J. M.Cancer Res. 1999. 59:578–585.
(16). Chiang Y.M.., Chuang D.Y.., Wang S.Y.., Kuo Y.H.., Tsai P.W.., Shyur L.F. J.Ethnopharmacol. 2004. 95:409–419.
(17). Yu H.L.., Xu J.H.., Lu W.Y.., Lin G.Q.Enzyme Microb. Tech. 2007. 40:354–361.
(18). Yang W.Q.., Qin X.D.., Shao H.J.., Fang L.Z.., Wang F.., Ding Z.H.., Dong Z.J.., Liu J.K. J.Basic Microbiol. 2007. 47:191–193.
(19). Boger D. L.., Panek J. S. J.Org. Chem. 1981. 46:1208–1210.
(20). Faizi S.., Ali M.., Saleem R.., Irfanullah ., Bibi S.Magn. Reson. Chem. 2001. 39:399–4. -21405.

(21). Choudhary A.., Kumar R.., Srivastava R. B.., Surapaneni S. K.., Tikoo K.., Singh I. P. J.Funct. Foods. 2015. 16:183–193.
(22). Watson C. Y.., Whish W. J. D.., Threadgill M. D.Bioorg. Med. Chem. 1998. 6:721–734.
(23). Akihisa T.., Tochizawa S.., Takahashi N.., Yamamoto A.., Zhang J.., Kikuchi T.., Fukatsu M.., Tokuda H.., Suzuki N.Chem. Biodivers. 2012. 9:1172–1187.
Go to : 
Table 1.
Inhibition effects of compounds isolated from fermented noni juice on the TNF-α-induced NF-κB and NO production, and Quinone Reductacse-1 (QR-1) inducing activities
| Compounds | Nitrite assay | NF-κB | QR1 | ||||
|---|---|---|---|---|---|---|---|
| % inhib.a | % surv.b | IC50 (μM) | % inhib.c | % surv.d | IC50 (μM) | IR | |
| 1 | 5.3 ± 1.3 | 103.4 ± 2.8 | nde | 56.0 ± 2.1 | 87 ± 4.1 | nd | 0.8 |
| 2 | 13.3 ± 1.3 | 79.6 ± 2.8 | nd | 0 | 107.6 ± 2.8 | nd | 1.4 |
| 3 | 3.7 ± 0.1 | 104.2 ± 0.4 | nd | 28.0 ± 2.0 | 88.0 ± 4.0 | nd | 1.8 |
| 4 | −f | − | − | − | − | − | − |
| 5 | −f | − | − | − | − | − | − |
| L-NMMAg | 25.1 ± 2.3 | ||||||
| TPCKh | 3.8 ± 0.6 | ||||||
| BAY-11h | 2.0 ± 0.3 | ||||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download