Abstract
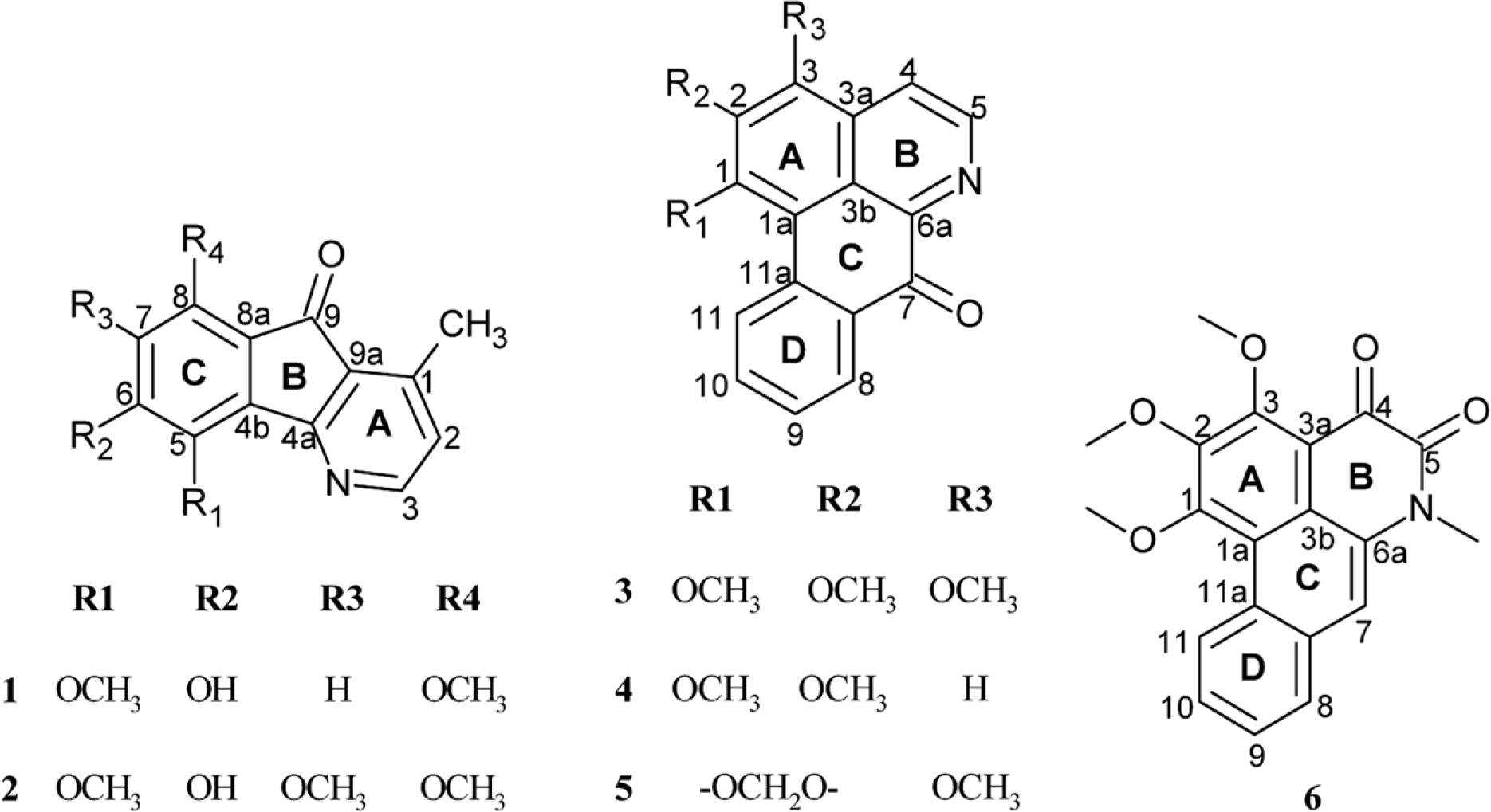
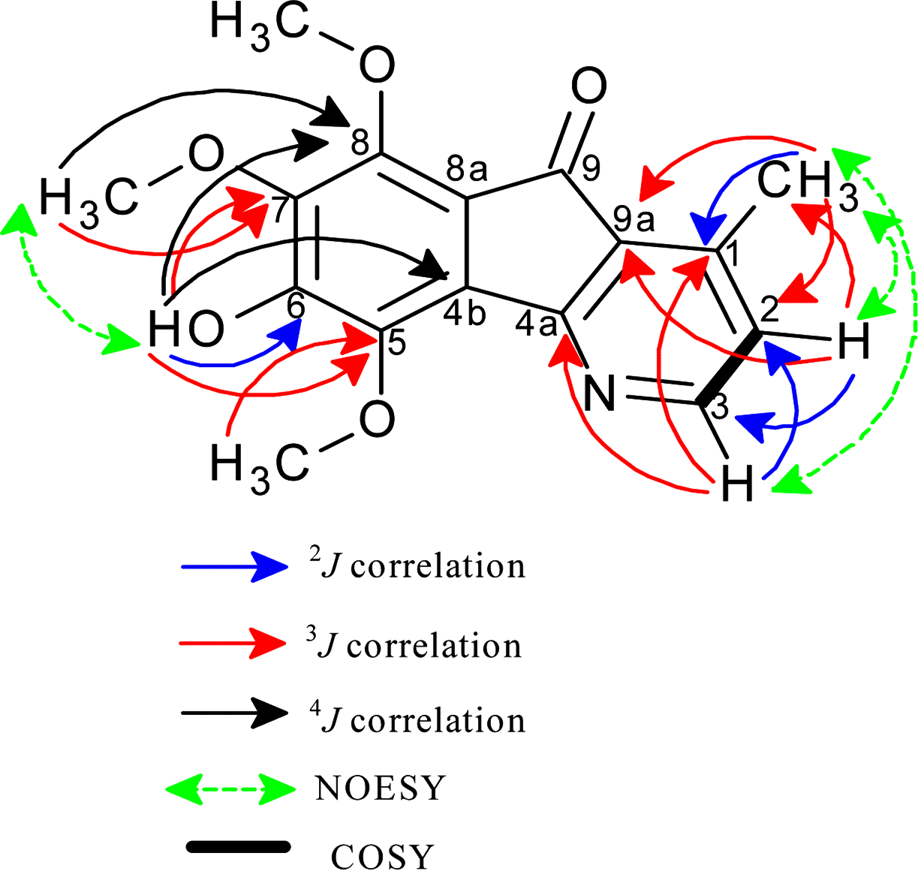
A phytochemical study of Alphonsea cylindrica King (unreported) has led to the isolation of six alkaloids. The compounds were identified as kinabaline (1; azafluorenone alkaloid), muniranine (2), O-methylmoschatoline (3; oxoaporphine alkaloid), lysicamine (4), atherospermidine (5) and N-methylouregidione (6; 4, 5-dioxoaporphine alkaloid). The structures of the isolated compounds were determined based on the spectroscopic techniques and by comparison with data reported in the literature. Alkaloid 2 was isolated as a new derivative of azafluorenone while alkaloids 1, 3 – 6 were isolated for the first time from Alphonsea species. In addition, alkaloid 3 and 4 showed inhibition zone against Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus cereus in disc diffusion test. The minimum inhibition concentration (MIC) values of lysicamine (4) against S. aureus, B. cereus and P. aeruginosa were found to be smaller than O-methylmoschatoline (3). Therefore, the reported antibacterial activity showed the potential of this plant as natural antibacterial agent and supported the documented traditional use of Alphonsea sp. in the treatment of diarrhea and fever.
Go to : 
References
(1). Srivastava G.., Mehrotra R. C.PLoS ONE. 2013. 8:1–6.
(2). Turner I. M.Gard. Bull. Singapore. 2009. 61:185–188.
(3). Latiff A.Malay. Nat. J. 2013. 65:247–273.
(4). Turner, I. M; Utteridge T. M. A.Blumea. 2015. 59:206–208.
(5). Turner I. M.Gard. Bull. Singapore. 2016. 68:65–69.
(6). IUCN Red List of Threatened Species. 2010.
(7). Batugal P. A.., Jayashree K.., Lee S. Y.., Jeffrey T. O.Medicinal Plants Research in Asia, Volume 1: The Framework and Project Work plans. International Plant Genetic Resources Institute –Regional Office for Asia; the Pacific and Oceania (IPGRI-APO), Malaysia,. 2004. 3–6.
(8). Jalil J.., Teh C. H.., Hussain K.., Jamal J. A.., Mohamad H. F.., Muhammad K.Abstract of International Conference on Natural Products. 2015. 154.
(9). Thang T. D.., Huong L. T.., Dai D. N.., Oguwande I. A.Nat. Prod. Res. 2013. 27:2022–2026.
(10). Johnson T. A.., Sohn J.., Ward A. E.., Cohen T. L.., Lorig-Roach N. D.., Chen H.., Pilli R. A.., Widjaja E. A.., Hanafi M.., Kardono L. B. S.., Lotulung P. D.., Boundy-Mills K.., Bjeldanes L. F.Bioorg. Med. Chem. 2013. 21:4358–4364.
(11). Xie N.., Xu R.., Zhong S.., Zhao S.Journal-china Pharmaceutical University,. 1994. 25:205.
(13). Xie N.., Zhong S.., Zhao S.., Peter G. W. J.China Pharm. Uni. 1989. 20:321–324.
(14). Yang N.., Xie N.., Zhi F. J.China Pharm. Uni. 1999. 30:171–173.
(15). Yang N. Y.., Xie N.., Kong L. Y.., Li G.Chin. Chem. Lett. 2000. 11:409.
(16). Ning X.., Yang N. Y.Chin. Chem. Lett. 1999. 10:671–672.
(17). Mahanta P. K.., Mathur R. K.., Gopinath K. W.Indian J. Chem. 1975. 13:306–308.
(18). Tadi D.., Wanningama G. P.., Cassels B. K.., Cavé A. J.Nat. cí Prod. 1987. 50:518–519.
(19). Gopinath K. W.., Mahanta P. K.., Bohlmann F.., Zedro C.Tetrahedron. 1976. 32:737–740.
(20). Narendra P. D.Res. J. Pharmacol. Pharmacodyn. 2009. 1:66–69.
(21). Horgen F. D.., Edrada R. A.., de los Reyes G.., Agcaoili F.., Madulid D. A.., Wongpanich V.., Angerhofer C. K.., Pezzuto J. M.., Soejarto D. D.., Farnsworth N. R.Phytomedicine. 2001. 8:71–81.
(22). Indrani V.., Madhuri T.., Lakshmi K. B.., Suvarnalatha D. P.Int. J. Sci. Res. Management. 2015. 3:2103–2105.
(23). Norhayati I.., Getha K.., Haffiz J. M.., Ilham A. M.., Sahira H. L.., Siti Syarifah M. M.., Syamil M. A. J.Trop. For. Sci. 2013. 25:52–59.
(24). Hanum F. I.., Ibrahim A. Z.., Khamis S.., Nazre M.., Lepun P.., Rusea G.., Lajuni J. J.., Latiff A. Pertanika J.Trop. Agric. Sci. 2001. 24:63–78.
(25). Sati S. C.., Khulbe K.., Joshi S.Res. J. Microbiol. 2011. 6:289–296.
(26). European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Clin. Microbiol. Infect. 2000. 9:1–7.
(27). Yusof H.., Din L. B.., Yaacob W. A.., Ibrahim N.., Yamin B. M.., Latiff A.Sains Malaysiana. 2015. 44:1125–1128.
(28). Husain K.., Jamal J. A.., Jalil J.Int. J. Pharm. Pharm. Sci. 2012. 4:465–467.
(29). Costa E. V.., Marques F. D. A.., Pinheiro M. L. B.., Braga R. M.., Delarmelina C.., Duarte M. C. T.., Ruiz A. L. T. G.., V; de Carvalho J. E.., Maia B. H. J.Braz. Chem. Soc. 2011. 22:1111–1117.
(30). Wirasathien L.., Boonarkart C.., Pengsuparp T.., Suttisri R.Pharm. Biol. 2006. 44:274–278.
(31). Tadi D.., Cassels B. K.., Leboeuf M.., Cavé A.Phytochemistry cí. 1987. 26:537–541.
(32). Yoshida N. C.., de Siqueira J. M.., Rodrigues R. P.., Correia R. P.., Garcez W. S. J.Braz. Chem. Soc. 2013. 24:529–533.
(33). Tadi D.., Cassels B. K.., Cavé A.Heterocycles. 1988. 27:407–c. í 421.
(34). Tan K. K.., Khoo T. J.., Rajagopal M.., Wiart C.Nat. Prod. Res. 2015. 29:2346–2349.
(35). Omar H.., Hashim N. M.., Zajmi A.., Nordin N.., Abdelwahab S. I.., Azizan A. H.., Hadi, A. H; Ali H. M.Molecules. 2013. 18:8994–9009.
(36). Tavares Lde C.., Zanon G.., Weber A. D.., Neto A. T.., Mostardeiro C. P.., Da Cruz I. B.., Oliveira R. M.., Ilha V.., Dalcol I. I.., Morel A. F.PLoS One. 2014. 9:, e97000.
Go to : 
Table 1.
NMR spectroscopic data for alkaloids 1 and 2 in CDCl3 (500 MHz 1H and 125 MHz 13C)
Table 2.
Diameter zone of inhibition for alkaloids 1 – 6 against S. aureus, B. cereus, S.typhi, E. coli and P. aeruginosa




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download