Abstract
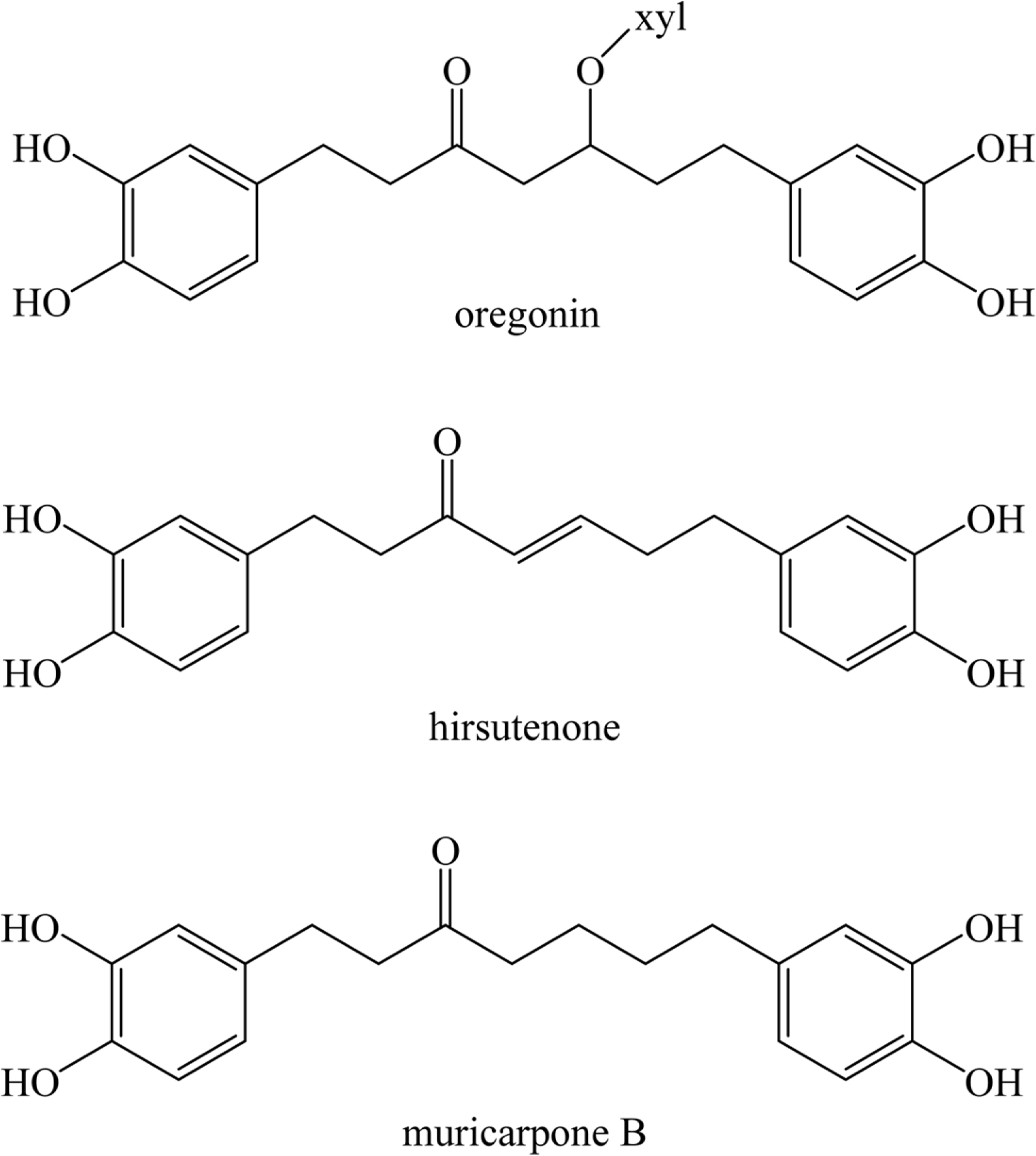
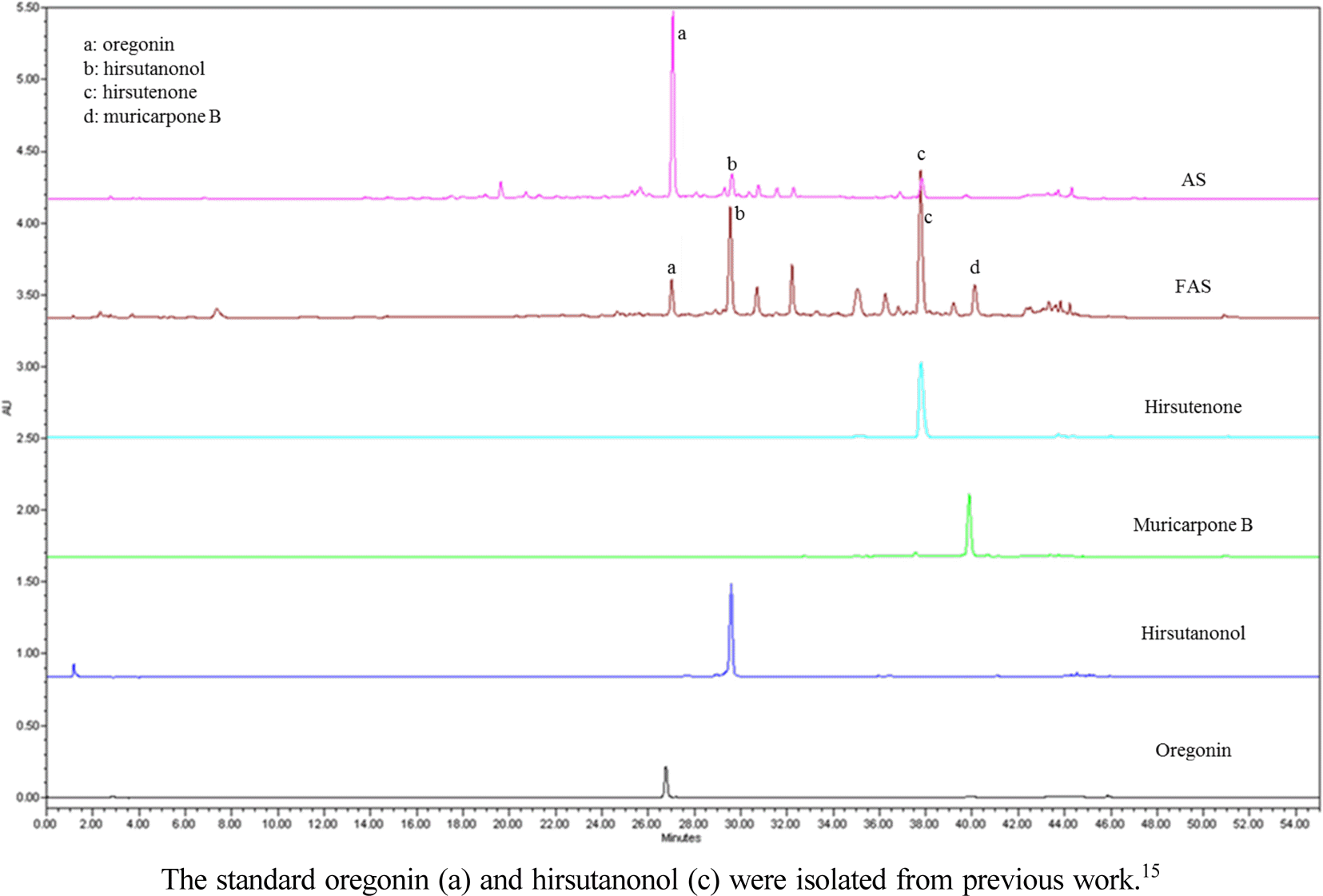
Alnus sibirica (AS) geographically distributes in Korea, Japan, Northeast China and Russia. The bark of this plant had been used for antipyretic, expectorant, anti-phlogistic, antitussive, anti-asthmatic and as a health tea for alcoholism. Recently, we studied various biological activities of AS and the isolated diarylheptanoid. In present study, we conducted fermentation of AS (FAS) and isolated two diarylheptanoid (hirsutenone and muricarpone B). Moreover, we established the validation and contents determinations of the two compounds by HPLC on FAS.
Go to : 
References
(1). Lee S. J.Korea folk Medicine; Seoul National University Publishing Center: Korea,. 1996. , pp. 40.
(2). Asakawa Y.., Genjida F.., Hayashi S.., Matsuura T.Tetrahedron Lett. 1969. 10:3235–3237.
(3). Terazawa M.., Okuyama H.., Miyake M.Mokuzai gakkaish. 1973. 19:45–46.
(4). Karchesy J. J.., Laver M. L.., Barofsky D. F.., Barofsky E. J.Chem. Soc. 1974. 16:649–650.
(5). Nomura M.., Tokoroyama T.., Kubota T.Phytochemistry. 1981. 20:1097–1104.
(6). Suga T.., Iwata N.., Asakawa Y.Bull. Chem. Soc. Jpn. 1972. 45:2058–2060.
(7). Asakawa Y.Bull. Chem. Soc. Japan. 1971. 44:2761–2766.
(8). Yoshida T.., Memon M. U.., Okuda T.Heterocycles. 1981. 16:1085–1088.
(9). Ishimatsu M.., Tanaka T.., Nonaka G.., Nishioka I.Phytochemistry. 1989. 28:3179–3184.
(10). Khvorst O. P.., Serbin A. G.., Komissarenko N. F.., Goldienko V. G.Khim. Farm. Zh. 1989. 23:445–449.
(11). Lee M. W.., Tanaka T.., Nonaka G.., Nishioka I.Phytochemistry. 1992. 31:967–970.
(12). Aoki T.., Ohta S.., Aratani S.., Hirata T.., Suga T. J.Chem. Soc. Perkin Trans. 1. 1982. 2:1399–1403.
(13). Sakamura F.., Ohta S.., Aoki T.., Syga T.Phytochemistry. 1985. 24:2744–2745.
(14). Suga T.., Ohta S.., Ohta E.., Aoki T.Phytochemistry. 1986. 25:1243–1244.
(15). Kim M. H.., Park K. H.., Kim S. R.., Park K. J.., Oh M. H.., Heo J. H.., Yoon K. H.., Yin J.., Yoon K. H.., Lee M. W.Nat. Prod. Res. 2016. 30:206–213.
(16). Bae C. I.., Gong J. M.., Oh J. W.., Kim H. J.., Oh G. J.., Park S. K.., Chung S. G.., Cho E. H.Yakhak Hoeji. 1997. 41:559–564.
(17). Lee M.., Song J. Y.., Chin Y. W.., Sung S. H.Bioorg. Med. Chem. Lett. 2013. 23:2069–2073.
(18). Pahng S. H.Korean J. Orient. Med. 2011. 17:53–60.
(19). Kim K. Y.., Song H. J.Herbal processology; Shinil Press: Korea,. 2002. , p. 547.
(20). Park J. H.., Kim H. J.., Lee M. J. J.Soc. Korean Med. Obes. Res. 2009. 9:1–14.
(21). Kim H. J.., Kim K. H.., Yeom S. H.., Kim M. K.., Shin J. G.., Lim H. W.., Lee M. W.Chinese Chem Lett. 2005. 16:1337–1340.
(22). Giang P. M.., Son P. T.., Matsunami K.., Otsuka H.Chem. Pharm. Bull. 2006. 54:139–140.
(23). Kuroyanagi M.., Shimomae M.., Nagashima Y.., Muto N.., Okuda T.., Kawahara N.., Nakane T.., Sano T.Chem. Pharm. Bull. 2005. 53:1519–1523.
(24). Jeong M. S.., Choi S. E.., Kim J. Y.., Kim J. S.., Kim E. J.., Park K. H.., Lee D. I.., Joo S. S.., Lee C. S.., Bang H.., Lee M. K.., Choi Y. W.., Li K. S.., Moon N. J.., Lee M. W.., Seo S. J.Clin. Dev. Immunol. 2010. 2010:618517.
(25). Kim J. H.., Lee K. W.., Lee M. W.., Lee H. J.., Kim S. H.., Surh Y. J.FEBS Lett. 2006. 580:385–392.
(26). Choi J. G.., Lee M. W.., Choi S. E.., Kim M. H.., Kang O. H.., Lee Y. S.., Chae H. S.., Obiang-Obounou B.., Oh Y. C.., Kim M. R.., Shin D. W.., Lee J. H.., Kwon D. Y.Eur. Rev. Med. Pharmacol. Sci. 2012. 16:853–859.
Go to : 
Table 1.
Analysis condition of HPLC
| Column | Thermo® Syncronis C18 HPLC column (5 µm, 250 × 4.6 mm) | ||||||
|---|---|---|---|---|---|---|---|
| Detector | Waters 112 UV/VIS (280 nm) | ||||||
| 0 min | 20 min | 35 min | 38 min | 45 min | 47 min | 55 min | |
| H2O | 95 | 75 | 60 | 0 | 0 | 95 | 95 |
| ACN | 5 | 25 | 40 | 100 | 100 | 5 | 5 |
Table 2.
Content of hirsutenone and muricarpone B in extract of FAS and AS
| Compound | FAS (%) | AS (%) |
|---|---|---|
| Hirsutenone | 28.70 | 2.74 |
| Muricarpone B | 6.72 | − |
Table 3.
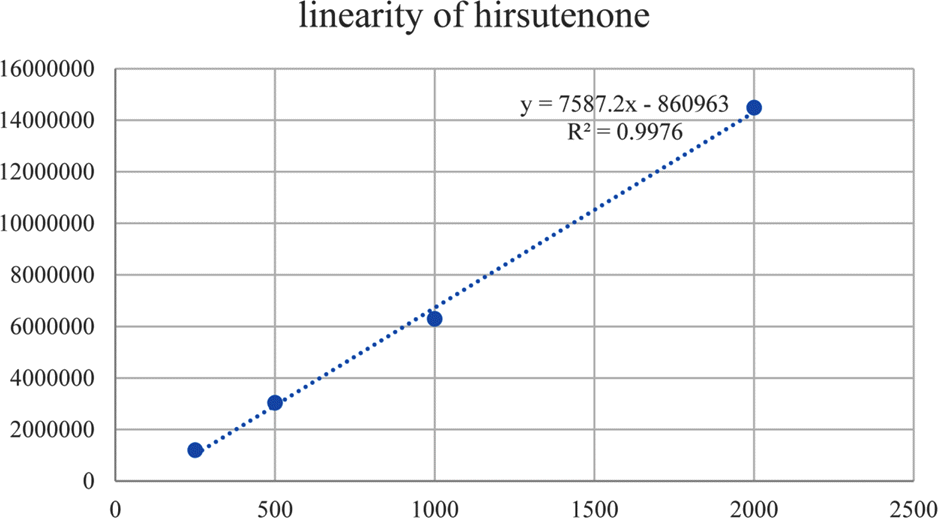
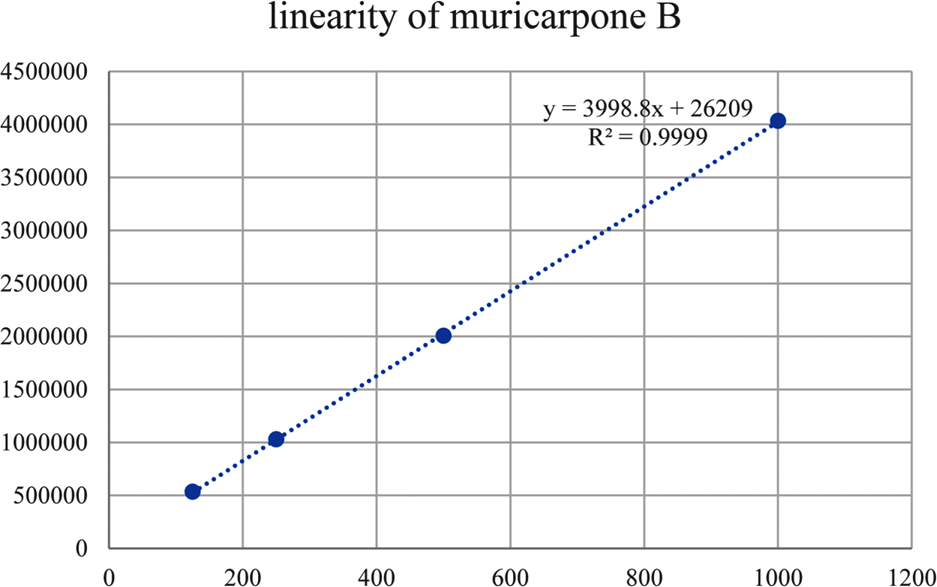
Linear ranges and LOQ of hirsutenone and muricarpone B
Table 4.
Precision and accuracy of hirsutenone and muricarpone B




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download