Abstract
This study was designed to investigate the synergetic hepatoprotective effects from a mixture of Korean Red Ginseng and Pueraria Radix on carbon tetrachloride (CCl4)-induced hepatotoxicity in mice. Liver toxicity was induced by intraperitoneal administration of CCl4 (0.6mg/kg) in 12groups of ICR mice. The negative control group was given CCl4 without test samples and the normal group was given no treatment. Among treatment groups, the RGAP treatment (Korean Red ginseng acetic acid extract: Pueraria radix water extract, w/ w, 38.4:57.6) decreased CCl4-elevated ALT (101.60 IU/L), AST (833.89 IU/L), and LDH (365.02 IU/L) levels in the serum, and increased the SOD (11.03 unit/mg protein) and CAT (0.37 unit/mg protein) levels and the LPO levels (59.09 µM/g tissue) more than that in the mice group with CCl4-induced control group hepatotoxicity. These results suggest that administration of a mixture of Korean Red ginseng and Pueraria radix decreases CCl4-induced liver damage and enhances antioxidant activity in mice and imply that administration of the mixture in a certain ratio is more effective than single administration of either Korean Red ginseng or Pueraria radix alone.
Go to : 
References
(1). Lee C. K.., Han Y. N.., Kim N. Y.., Choi J. W. J.Ginseng Res. 2003. 27:11–16.
(2). Kim H. J.., Lee J. W.., Ji Y. J.., Yu M. H.., Park J. H.., Lee K. D.., Lee I. S. J.Korean Soc. Food Sci. Nutr. 2007. 36:521–526.
(3). Campos R.., Garrido A.., Guerra R.., Valenzula A.Planta Med. 1989. 55:417–419.
(4). Han J. Y.., Lee S. K.., Yang J. H.., Kim S. J.., Sim J. H.., Kim M. G.., Jeong T. C.., Ku S. K.., Cho I. J.., Kim S. H. J.Ginseng Res. 2015. 39:105–115.
(5). Han B. H.., Park M. W.., Han Y. N.Arch. Pharm. Res. 1981. 4:53–58.
(6). Lee F. C.., Park J. K.., Kim E. K.., Ko J. K.., Lee J. S.., Kim K. Y.Proc 4th Int Ginseng Symp. 1984. 21–26.
(7). Ha T. Y.., Lee J. H.., Kim S. H. J.Korea Soc. Microbiol. 1986. 21:133–144.
(8). Lee S. E.., Lee S. W.., Bang J. K.., Yu Y. J.., Seong N. S.Korean J. Medicinal Crop Sci. 2004. 12:237–242.
(9). Shin H. R.., Kim J. Y.., Yun T. K.., Morgan G.., Vainio H.Cancer Causes Control. 2000. 11:565–576.

(10). Yun T. K.Lancet Oncol. 2001. 2:49–55.
(11). Nakamoto H.., Iwasaki Y.., Kizu H.Yakugaku Zasshi. 1977. 97:103–105.
(12). Miura K.., Takeda R.., Nakamoto H.., Saito H. J.Appl. Pharmacol. 1971. 5:247–252.
(13). Fan L. L.., Zeng G. Y.., Zhou Y. P.., Zhang L. Y.., Cheng Y. S.Chin. Med. J. 1982. 95:145–150.
(14). Jha H. C.., von Recklinghausen G.., Zilliken F.Biochem. Pharmacol. 1985. 34:1367–1369.
(15). Lin R. C.., Guthrie S.., Xie C. Y.., Mai K.., Lee, D. Y, Lumeng L.., Li T. K.Alcohol. Clin. Exp. Res. 1996. 20:659–663.
(16). Lee C. H.., Han S. H.., Kim J. B.., Min S. G. J.Korean Soc. Food Sci. Nutr. 1995. 24:713–719.
(17). Reitman S.., Frankel S.Am. J. Clin. Pathol. 1957. 28:56–63.
(18). King J.Clin Chem. 1972. 18:1443.
(19). Cropo J. D.., McCord J. M.., Fridovich I.Methods. Enzymol. 1978. 53:382–393.
(20). Lee E.., Shin C. O.Korean J. Plant Res. 2007. 20:475–480.
(21). Ohkawa H.., Ohishi N.., Yagi K.Anal. Biochem. 1979. 95:351–358.
(22). Uchida T.., Kao H.., Quispe-Sjogen M.., Peters R. L.Gastroenterology. 1983. 84:683–692.
(23). Davies P.., Maloney A. J. F.Lancet. 1976. 2:1403–1407.
(24). Kim Y. S.Korean J. Ginseng Sci. 1995. 19:108–113.
(25). Lee J. S.., Kim E. S.., Kim S. W. J.Korean Soc. Food Sci. Nutr. 1999. 28:901–906.
(26). Lee Y. K. J.East Asian Soc. Diet. Life. 1994. 4:59–67.
(27). Seong G. S.., Chun S. G.., Chang C. C. J.Ginseng Res. 2005. 29:131–137.
(28). Sung K. S.., Chun C.., Kwon Y. H.., Chang C. C. J.Ginseng Res. 2000. 24:176–182.
(29). Nam K. Y.., Choi J. E.., Park J. D.Korean J. Medicinal Crop Sci. 2013. 21:401–414.
(30). Kong Y. H.., Rho J. H.., Cho C. W.., Kim M. H.., Lee Y. C.., Kim S. S.., Lee P. J.., Choi S. Y. J.Ginseng Res. 2009. 33:194–198.
(31). Bae E. A.., Han M. J.., Kim E. J.., Kim D. H.Arch. Pharm. Res. 2004. 27:61–67.
(32). Kim M. H.., Hong H. D.., Kim Y. C.., Rhee Y. K.., Kim K. T.., Rho J. H. J.Ginseng Res. 2010. 34:93–97.
(33). Hong H. D.., Sim E. M.., Kim K.., Rho J.., Rhee Y. K.., Cho C. W.Food Sci. Biotechnol. 2009. 18:565–569.
Go to : 
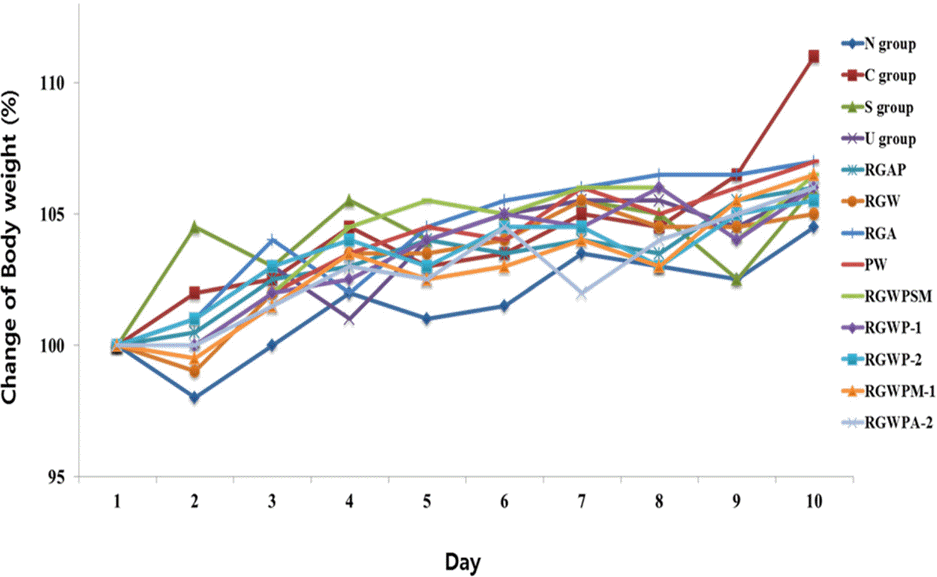 | Fig. 1.Body weight change after 10-day oral administration of Red ginseng and Pueraria radix extract 1) Refer to Table 1. 2) Data are expressed as the mean ± SE (n = 7) |
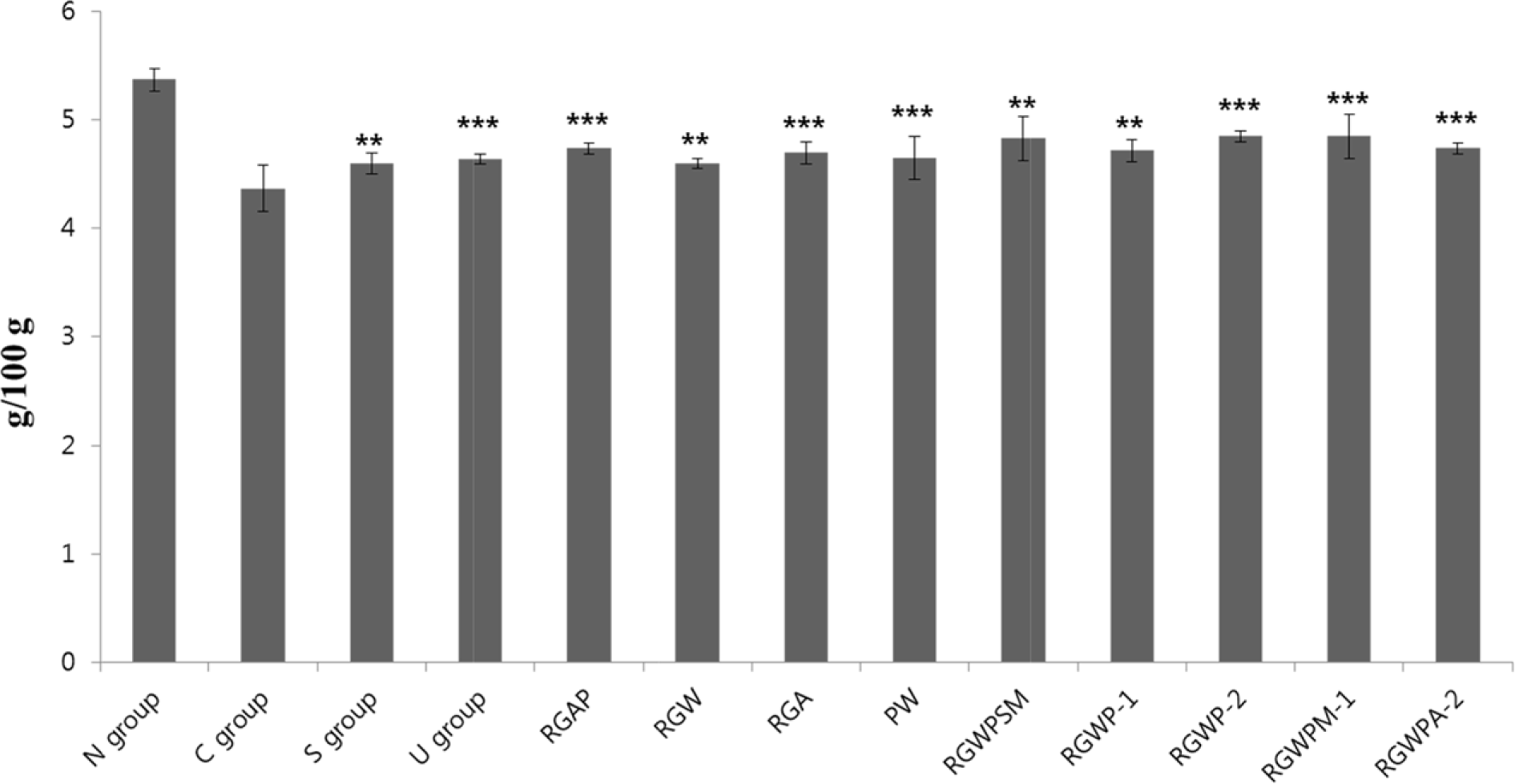 | Fig. 2.Liver weight per 100 g of body weight, to examine the effect of CCl4 and administration of extract of Red ginseng and Pueraria radix on the weight of the organ 1) Refer to Table 1. 2) Data are expressed as the mean ± SE (n = 7) Statistical significance of differences was calculated between C group and experiment groups. ∗P <0.05, ∗∗P <0.01, ∗∗∗P <0.001. |
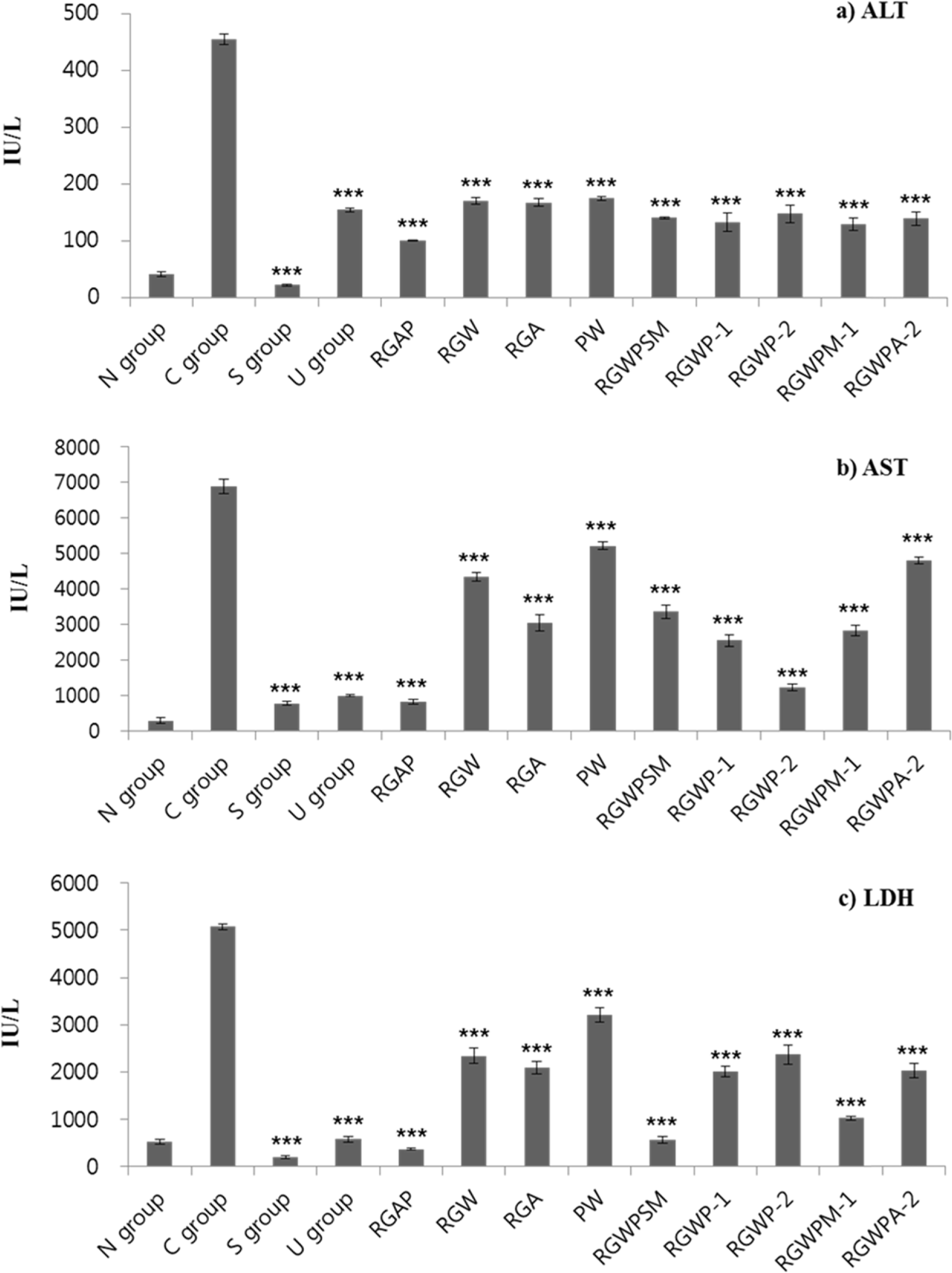 | Fig. 3.Effect of Red ginseng and Pueraria radix on the activity of serum alanine transaminase (a; ALT), aspartate transaminase (b; AST), and lactate dehydrogenase (c; LDH) in CCl4-treated ICR mice 1) Refer to Table 1 2) Data are expressed as the mean ± SE (n = 7) Statistical significance of differences was calculated between C group and experiment groups. ∗P <0.05, ∗∗P <0.01, ∗∗∗P <0.001. |
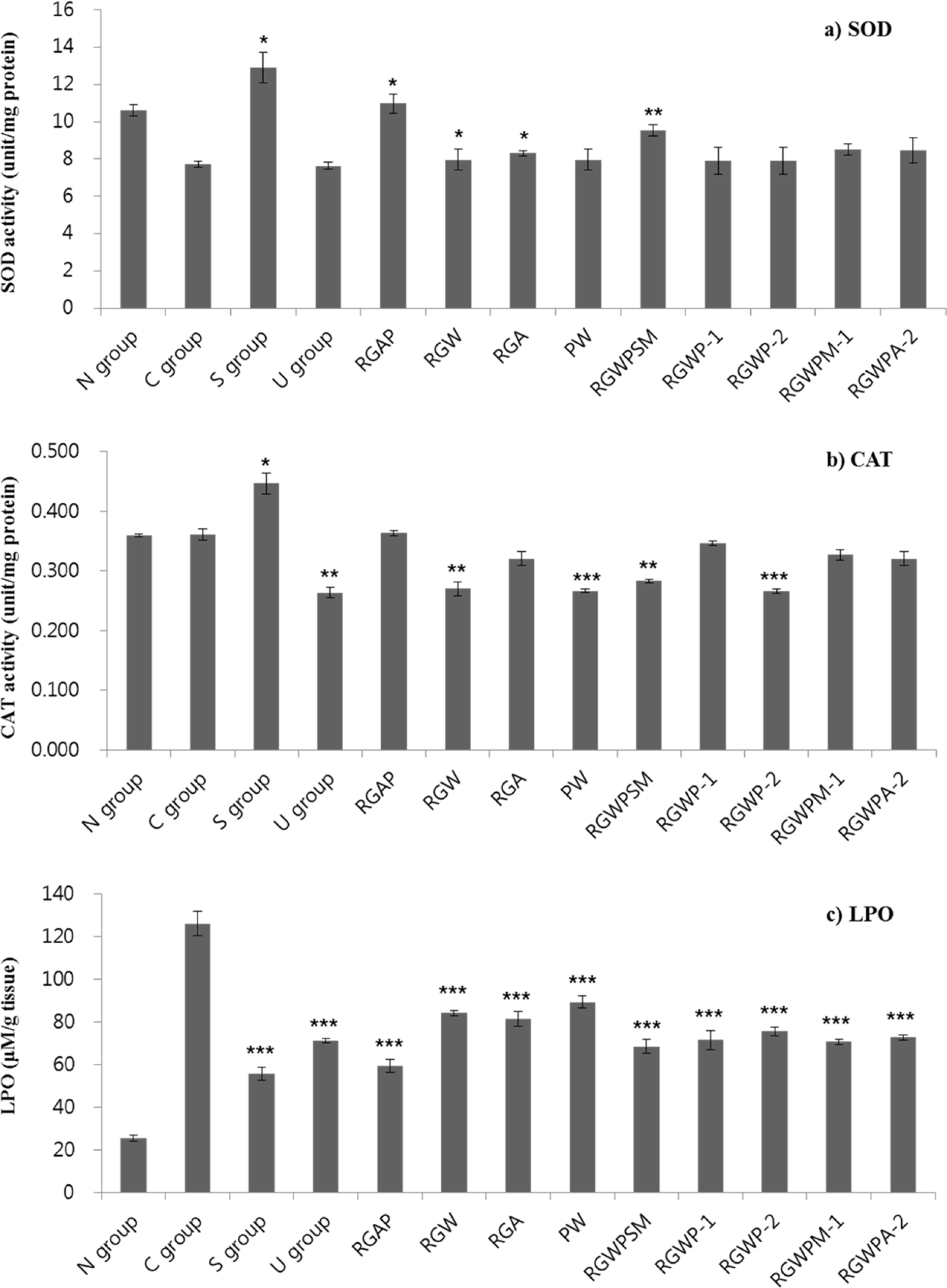 | Fig. 4.Effect of Red ginseng and Pueraria radix on the activity of superoxide dismutase (a; SOD), catalase (b; CAT), and lipid peroxidase (c; LPO) in CCl4-treated ICR mice 1) Refer to Table 1 2) Data are expressed as the mean ± SE (n = 7) Statistical significance of differences was calculated between C group and experiment groups. ∗P <0.05, ∗∗P <0.01, ∗∗∗P <0.001. |
Table 1.
Composition of oral administration experimental diets (%)
| Ingredients | Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RGAP | RGW | RGA | PW | RGWPSM | RGWP-1 | RGWP-2 | RGWPM-1 | RGWPA-2 | |
| RGAE1) | 38.4 | − | 96.0 | − | − | − | − | − | − |
| RGWE2) | − | 96.0 | − | − | 38.4 | 19.2 | 38.4 | 38.4 | 38.4 |
| PWE3) | 57.6 | − | − | 96.0 | 19.2 | 76.8 | 57.6 | 45.1 | 45.1 |
| Vitamin complex | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| A. asiatica | − | − | − | − | − | − | − | − | 12.5 |
| Milk thistle | − | − | − | − | − | − | − | 12.5 | − |
| Shell extract | − | − | − | − | 19.2 | − | − | − | − |
| M. lusoria | − | − | − | − | 19.2 | − | − | − | − |
| Total (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download