Abstract
The isolation of the MeOH extract from the flower bud of Magnolia biondii Pamp. using various column chromatographies and HPLC led to eleven neoglignan derivatives (1 – 11). Their structures were mainly determined by 1D and 2D NMR spectral data analysis and physiological methods. The isolated compounds (1 – 11) were tested for anti-allergic effects using IL-2 inhibitory assay in Jurkat T cells.
Go to : 
References
(1). Park C. S.., Kim T. B.., Lee J. Y.., Park J. Y.., Lee Y. C.., Jeong S. S.., Lee Y. D.., Cho Y. S.., Moon H. B.Korean J. Intern. Med. 2012. 27:84–90.
(2). Song Q.., Fischer N. H.Rev. Soc. Quím. Mex. 1999. 43:211–218.
(3). Marin G. H.., Mansilla E.., Ciocchini S.., Quiroga N.., Cardozo N.., Weiss M.., Gustavo H. M.., Trebucq H.Annalen der Chemischen Forschung. 2013. 1:50–55.
(4). Lee S.., Chappell J.Plant Physiol. 2008. 147:1017–1033.
(5). Haraguchi H.., Ishikawa H.., Shirataki N.., Fukuda A. J.Pharm. Pharmacol. 1997. 49:209–212.
(6). Syu W. J.., Shen C. C.., Lu J. J.., Lee G. H.., Sun C. M.Chem. Biodivers. 2004. 1:530–537.
(7). Kuo W. L.., Chung C. Y.., Hwang T. L.., Chen J. J.Phytochemistry. 2013. 85:153–160.
(8). Akber U.., Na B. R.., Ko Y. S.., Lee H. S.., Kim H. R.., Kwon M. S.., Park Z. Y.., Choi E. J.., Han W. C.., Lee S. H.., Oh H. M.., Jun C. D.Int. Immunopharmacol. 2015. 25:130–140.
(9). Hershko A. Y.., Suzuki R.., Charles N.., Alvarez-Errico D.., Sargent J. L.., Laurence A.., Rivera J.Immunity. 2011. 35:562–571.
(10). Chen C. C.., Huang Y. L.., Chen Y. P.., Hsu H. Y.., Kuo Y. H.Chem. Pharm. Bull. 1988. 36:1791–1795.
(11). Tyagi O. D.., Jensen S.., Boll P. M.., Sharma N. K.., Bisht K. S.., Parmar V. S.Phytochemistry. 1993. 32:445–448.
(12). Ponpipom M. M.., Bugianesi R. L.., Brooker D. R.., Yue B. Z.., Hwang S. B.., Shen T. Y. J.Med. Chem. 1987. 30:136–142.
(13). Wenkert E.., Gottlieb H. E.., Gottlieb O. R.., Pereira M. O. S.., Formiga M. D.Phytochemistry. 1976. 15:1547–1551.
(14). Jensen S.., Olsen C. E.., Tyagi O. D.., Boll P. M.., Hussaini F. A.., Gupta S.., Bisht K. S.., Parmar V. S.Phytochemistry. 1994. 36:789–792.
(15). Tyagi O. D.., Wengel J.., Prasad A. K.., Boll P. M.., Olsen C. E.., Pati H. N.., Bisht K. S.., Parmar V. S.Acta Chem. Scand. 1994. 48:1007–1011.
(16). El-Feraly F. S.., Cheatham S. F.., Hufford C. D.., Li W. S.Phytochemistry. 1982. 21:1133–1135.
(17). Pieters L.., de Bruyne T.., Claeys M.., Vlietinck A.., Calomme M.., vanden Berghe D. J.Nat. Prod. 1993. 56:899–906.
(18). Kuroyanagi M.., Yoshida K.., Yamamoto A.., Miwa M.Chem. Pharm. Bull. 2000. 48:832–837.
(19). Du J.., Wang M. L.., Chen R. Y.., Yu D. Q. J.Asian Nat. Prod. Res. 2001. 3:313–319.
(20). Kasperska-Zajac A.., Brzoza Z.., Rogala B.Recent Pat. Inflamm. Allergy Drug Discov. 2008. 2:72–76.
(21). Noshita T.., Funayama S.., Hirakawa T.., Kidachi Y.., Ryoyama K.Biosci. Biotechnol. Biochem. 2008. 72:2775–2778.
(22). Reddy S. D.., Siva B.., Poornima B.., Kumar D. A.., Tiwari A. K.., Ramesh U.., Babu K. S.Pharmacogn. Mag. 2015. 11:235–241.
Go to : 
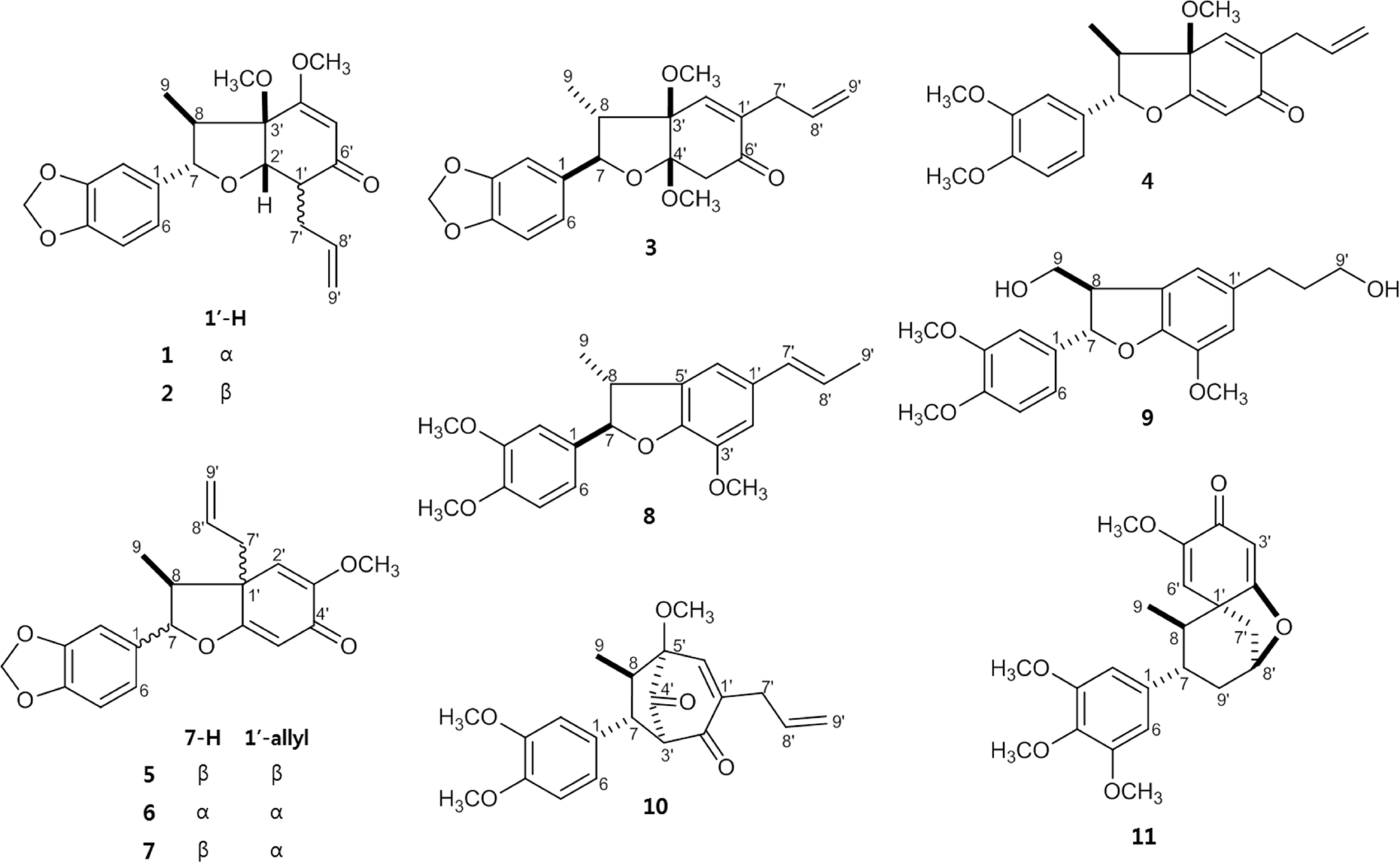 | Fig. 1.Chemical structures of neolignans 1 – 11 isolated from the flower of Magnolia biondii Pamp. |
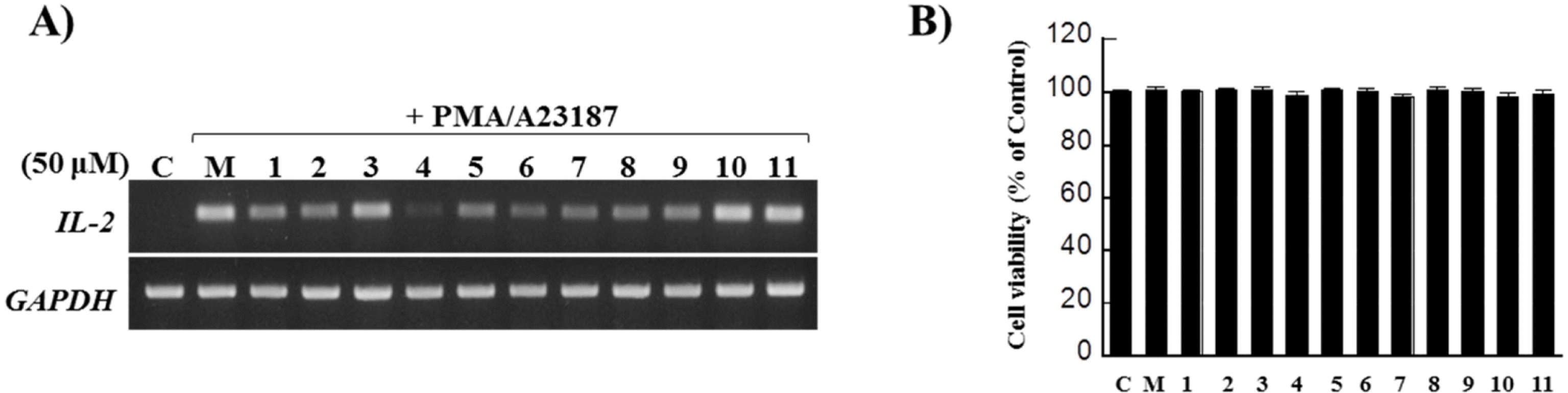 | Fig. 2.Inhibition of IL-2 expression and cell viability by neolignans 1–11 isolated from M. biondii. A) Jurkat T cells (1 × 106) were treated with 50 µM concentrations of 1 – 11 for 30 min and stimulated with PMA (100 nM)/A23187 (1 µM) for 6 h. After incubation, cells were harvested and total RNA was isolated from harvested cells. Human IL-2 mRNA levels were detected by conventional PCR. B) Jurkat T cells (3×105) were seeded in a 24 well-plate and incubated with 50 µM concentrations of 1–11 for 24 h. After incubation, cell viability was examined by MTT assay. Data was shown as percentages compared to cell viability treated with Mock control. |
Table 1.
1H NMR spectroscopic data for compounds 1 – 7 (δ values)a
| Position | 1b | 2b | 3c | 4c | 5c | 6c | 7c |
|---|---|---|---|---|---|---|---|
| 2 | 6.81, s | 6.82, d (1.5) | 6.78, m | 6.92, m | |||
| 5 | 6.77, brs | 6.70, m | 6.78, d (8.0) | 6.96, m | 6.85, m | 6.84, d (8.4) | 6.87, d (7.9) |
| 6 | 6.75, dd (8.0, 1.5) | 6.78, m | 6.92, m | ||||
| 7 | 4.70, d (10.3) | 4.24, d (3.6) | 4.09, d (10.9) | 5.41, d (10.1) | 5.42, d (10.0) | 6.14, d (5.1) | 4.68, dd (17.0, 2.0) |
| 8 | 2.22, dd (10.3, 6.7) | 2.69, m | 2.73, m | 2.30, dq (9.5, 6.8) | 2.30, m | 2.83, dd (7.3, 5.1) | 2.82, q (7.2) |
| 9 | 1.07, d (6.7) | 1.06, d (7.0) | 0.97, d (7.0) | 1.15, d (6.8) | 1.17, d (6.9) | 0.50, d (7.3) | 1.21, d (7.2) |
| 1' | 2.74, m | 2.09, m | |||||
| 2' | 4.43, d (8.8) | 4.25, s | 6.42, s | 5.80, s | 5.84, s | 5.81, s | |
| 5' | 5.56, s | 5.71, s | 5.67, s | 5.77, s | 5.76, s | ||
| 2.46, dd | 2.68, dd | 2.23, dd | |||||
| 7' | 2.57, m, 2.74, m | 2.37, m, 2.86, m | 3.12, ddd (6.7, 2.5, 1.2) | 3.11, dd (6.8, 1.2), 3.16, dd (6.8, 1.2) | (13.6, 7.3), 2.68, dd | (13.5, 7.0), 2.77, dd | (13.4, 6.8), 2.35, dd |
| (13.6, 7.3) | (13.5, 7.8) | (13.4, 8.0) | |||||
| 8' | 5.90, m | 5.92, m | 6.54, d (1.2) | 5.92, ddt (16.9, 10.1, 6.9) | 5.53, ddt (17.3, 10.1, 7.3) | 5.72, m | 5.54, dddd (17.0, 10.2, 7.9, 6.8) |
| 9' | 5.10, dd (9.9, 1.5), 5.17, dd (17.3, 1.5) | 5.05, m, 5.15, dd (17.1, 1.5) | 5.14, m | 5.13, m | 5.04, ddd (18.8, 13.6, 1.9) | 5.14, t (12.2) | 5.00, dd (10.2, 2.0), 5.47, d (0.96) |
| –OCH2O- | – 5.95, s | 5.92, m | 5.95, s | 5.97, s | 5.96, s | 5.99, s | |
| 3-OCH3 | 3.82, s | ||||||
| 4-OCH3 | |||||||
| 3'-OCH3 | 3.21, s | 3.32, s | 3.19, s | 3.14, s | 3.69, s | 3.67, s | 3.67, s |
| 4'-OCH3 | 3.76, s | 3.73, s | 3.41, s | ||||
Table 2.
Table 3.
13C NMR spectroscopic data for compounds 1 – 11 (δ values)a
| Position | 1b | 2b | 3c | 4c | 5c | 6c | 7c | 8b | 9b | 10b | 11b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 134.4 | 134.2 | 135.5 | 131.3 | 133.1 | 133.2 | 136.4 | 132.8 | 133.9 | 134.0 | 139.1 |
| 2 | 106.9 | 106.7 | 108.8 | 111.2 | 107.8 | 107.3 | 106.0 | 109.7 | 109.5 | 110.2 | 104.8 |
| 3 | 148.0 | 147.9 | 149.5 | 151.3 | 149.8 | 149.4 | 149.7 | 149.3 | 149.3 | 148.6 | 153.4 |
| 4 | 147.6 | 147.5 | 149.1 | 150.8 | 149.6 | 148.8 | 148.6 | 146.8 | 149.1 | 149.6 | 137.0 |
| 5 | 108.2 | 108.1 | 108.1 | 112.8 | 109.1 | 109.2 | 109.3 | 110.0 | 111.1 | 111.7 | 153.4 |
| 6 | 120.5 | 120.1 | 122.2 | 121.2 | 120.2 | 120.4 | 120.3 | 119.4 | 118.8 | 119.5 | 104.8 |
| 7 | 87.2 | 86.1 | 86.8 | 93.3 | 92.8 | 89.3 | 94.9 | 93.8 | 87.9 | 49.0 | 45.4 |
| 8 | 51.4 | 48.5 | 50.6 | 51.5 | 37.1 | 44.2 | 46.4 | 45.8 | 53.9 | 45.5 | 37.9 |
| 9 | 8.9 | 11.2 | 9.4 | 6.9 | 8.3 | 12.4 | 18.8 | 17.8 | 64.1 | 13.8 | 14.6 |
| 1' | 52.8 | 54.1 | 144.5 | 143.9 | 53.2 | 55.8 | 55.0 | 132.4 | 135.5 | 140.6 | 50.4 |
| 2' | 80.0 | 81.1 | 140.2 | 133.6 | 111.1 | 112.3 | 112.1 | 109.4 | 112.6 | 194.5 | 180.0 |
| 3' | 84.7 | 82.3 | 83.3 | 79.3 | 154.3 | 153.8 | 153.7 | 144.3 | 144.3 | 70.0 | 101.5 |
| 4' | 172.4 | 173.4 | 103.4 | 177.4 | 185.7 | 185.5 | 185.4 | 149.3 | 146.7 | 202.4 | 183.1 |
| 5' | 105.7 | 103.5 | 44.2 | 102.9 | 102.8 | 102.6 | 102.7 | 133.4 | 127.9 | 89.5 | 153.4 |
| 6' | 196.4 | 196.9 | 196.1 | 189.2 | 185.3 | 185.1 | 185.3 | 113.5 | 116.1 | 147.3 | 108.9 |
| 7' | 31.3 | 29.2 | 34.5 | 34.6 | 50.9 | 46.5 | 46.7 | 131.1 | 32.1 | 32.9 | 60.9 |
| 8' | 135.4 | 136.4 | 136.4 | 136.6 | 132.6 | 132.2 | 132.9 | 123.7 | 34.7 | 133.9 | 81.9 |
| 9' | 117.9 | 117.0 | 117.6 | 117.5 | 122.1 | 120.2 | 118.4 | 18.5 | 62.4 | 118.3 | 43.7 |
| –OCH2O- | 101.2 | 101.2 | 102.5 | − | 101.9 | 102.2 | 102.7 | − | − | − | − |
| 3-OCH3 | − | − | − | 56.5 | − | − | − | 56.1 | 56.2 | 56.1 | 56.2 |
| 4-OCH3 | − | − | − | 56.5 | − | − | − | 56.1 | 56.2 | 56.1 | 60.9 |
| 5-OCH3 | − | − | − | − | − | − | − | − | − | − | 56.2 |
| 3'-OCH3 | 51.5 | 53.4 | 47.7 | 51.6 | 55.8 | 56.2 | 55.8 | 56.8 | 56.1 | − | − |
| 4'-OCH3 | 56.4 | 56.1 | 51.4 | − | − | − | − | − | − | − | − |
| 5'-OCH3 | − | − | − | − | − | − | − | − | − | 54.1 | 55.3 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download