Abstract
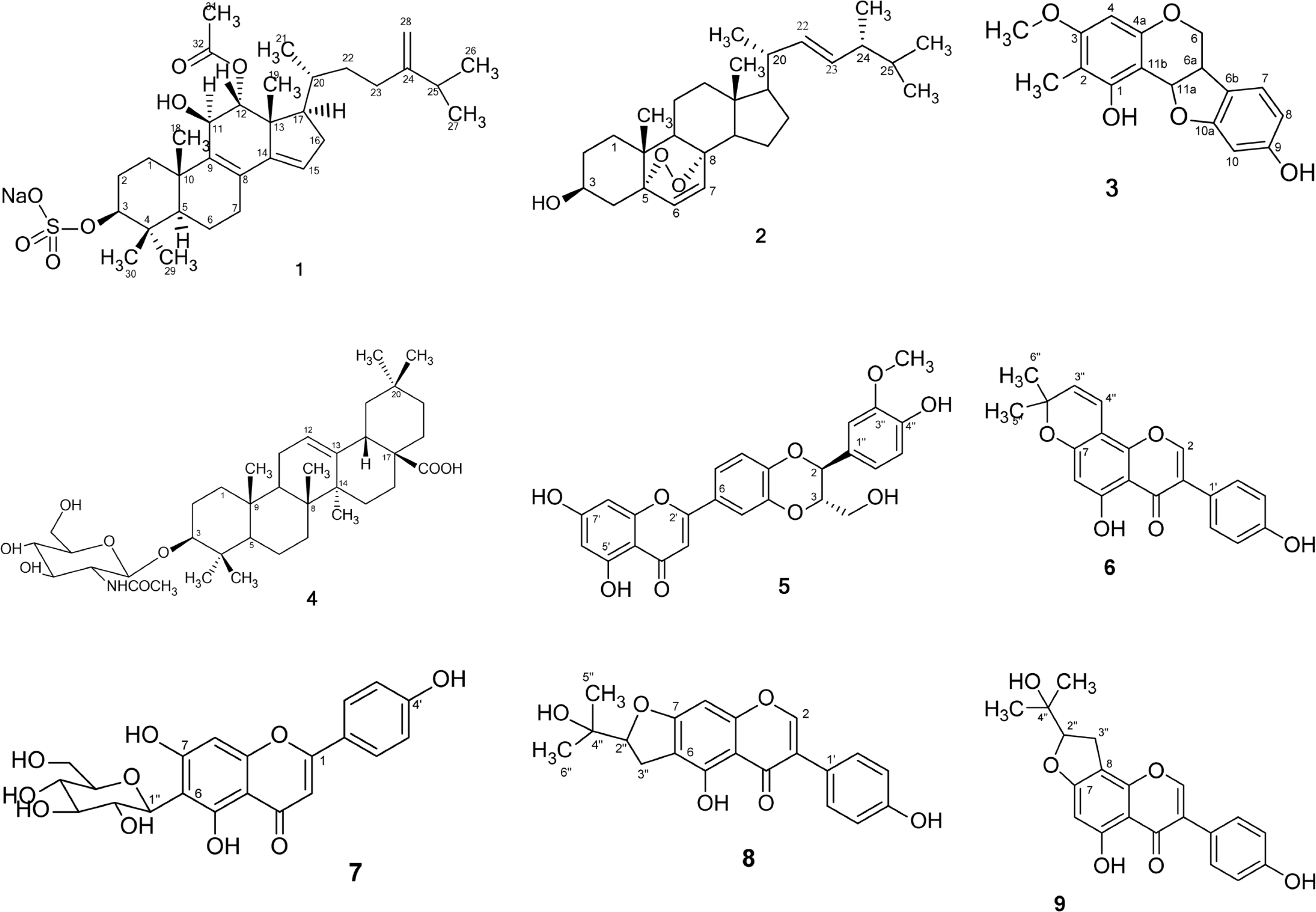
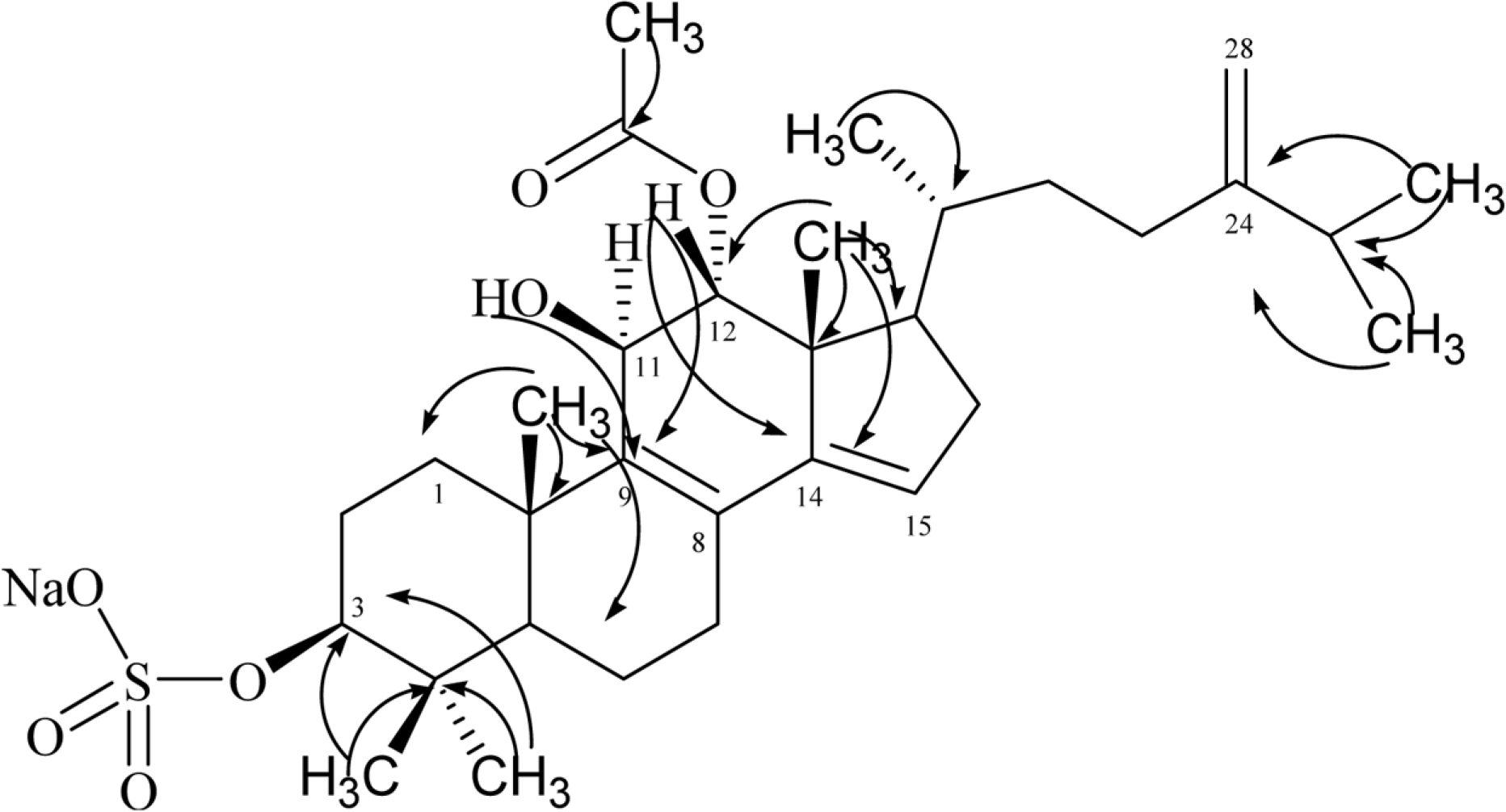
A new tetracyclic triterpenoid [4,4,24-trimethylcholesta-Δ8,9;14,15;24,28-trien-3β,11β,12α-triol-12-acetate, 3-sulfate] sodium salt (1), together with eight known compounds including ergosterol 5α,8α-endoperoxide (2), 1,9-dihydroxy-3-methoxy-2-methylpterocarpan (3), 3-O-β-D-2-acetyl-amino-2-deoxyglucopyranoxyloleanoic acid (4), hydnocarpin (5), derrone (6), isovitexin (7), erythrinin C (8), and 5,4'-dihydroxy-2''-hydroxyisopropyldihydrofurano [4,5:7,8]-isoflavone (9), were isolated from the EtOAc soluble fraction of the methanol extract of aerial part of Desmodium uncinatum collected in the western highland of Cameroon. The structures of these compounds were established by comprehensive interpretation of their spectral data mainly including 1D- (1H and13C), 2D-NMR (1H-1H COSY, HMQC, HMBC) spectroscopic and ESI-TOF-MS mass spectrometric analysis. The isolation of an integracide-like compound from plant origin is a very unusual finding.
Go to : 
References
(1). Liguo F.., Tanqing C.., Kaiyong L.., Hong T.., Lin Qi.Higher plants of China; Qingdao Publishing House: Qingdao,. 2008. , p. 152.
(2). Ma X.., Zheng C.., Hu C.., Rahman K.., Qin L. J.Ethnopharmacol. 2011. 138:314–332.
(3). Vedpal, Dhanabal S. P.., Dhamodaran P.., Chaitnya M. V. N. L.., Duraiswamy B.., Jayaram U.., Srivastava N. J.Chem. Pharm. Res. 2016. 8:91–97.
(4). Tsanuo M. K.., Hassanali A.., Hooper A. M.., Khan Z.., Kaberia F.., Pickett J. A.., Wadhams L. J.Phytochemistry. 2003. 64:265–273.
(5). Khan Z. R.., Hassanali A.., Overholt W.., Khamis T. M.., Hooper A. M.., Pickett J. A.., Wadhams L. J.., Woodcock C. M. J.Chem. Ecol. 2002. 28:1871–1885.

(6). Guchu S. M.., Yenesew A.., Tsanuo M. K.., Gikonyo N. K.., Pickett J. A.., Hooper A. M.., Hassanali A.Phytochemistry. 2007. 68:646–651.
(7). Correa E.., Quiñones W.., Torres F.., Cardona D.., Franco A. E.., Robledo S.., Echeverri F.Actual. Biol. 2005. 27:39–42.
(8). Wang P.., Wang J.., Guo T.., Li Y.Carbohydr. Res. 2010. 345:607–620.
(9). Afifi M. S. A.., Ahmed M. M.., Pezzuto J. M.., Kinghorn A. D.Phytochemistry. 1993. 34:839–841.
(10). Máximoa P.., Lourenço A.., Savluchinske Feio S.., Roseiro J. C. Z.Naturforsch. C. 2002. 57:609–613.
(11). Peng J.., Fan G.., Hong Z.., Chai Y.., Wu Y. J.Chromatogr. A. 2005. 1074:111–115.
(12). Tahara S.., Ingham J. L.., Mizutani J.Agric. Biol. Chem. 1985. 49:1775–1783.
(13). Dai J.., Shen D.., Yoshida W.Y.., Parrish S. M.., Williams P. G.Planta Med. 2012. 78:1357–1362.
(14). Brill G. M.., Kati W. M.., Montgomery D.., Karwowski J. P.., Humphrey P. E.., Jackson M.., Clement J. J.., Kadam S.., Chen R. H.., McAlpine J. B. J.Antibiot. 1996. 49:541–546.
(15). Singh S. B.., Zink D. L.., Dombrowski A. W.., Polishook J. D.., Ondeyka J. G.., Hirshfield J.., Felock P.., Hazuda D. J.Bioorg. Med. Chem. 2003. 11:1577–1582.
(16). Ibrahim S. R. M.., Mohamed G. A.., Ross S. A.Phytochem. Lett. 2016. 15:125–130.
(17). Ibrahim S. R. M.., Abdallah H. M.., Mohamed G. A.., Ross S. A.Fitoterapia. 2016. 112:161–167.
(18). Chatterjee S.., Sarkar A.., Dutta P. C. J.Chem. Soc., Perkin Trans. 1979. 1:2914–2919.
(19). Singh S. B.., Ondeyka J. G.., Schleif W. A.., Felock P.., Hazuda D. J. J.Nat. Prod. 2003. 66:1338–1344.
Go to : 
Table 1.
1H-NMR (400 MHz) and 13C-NMR (100 MHz) data of compound 1 (J in Hz)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download