Abstract
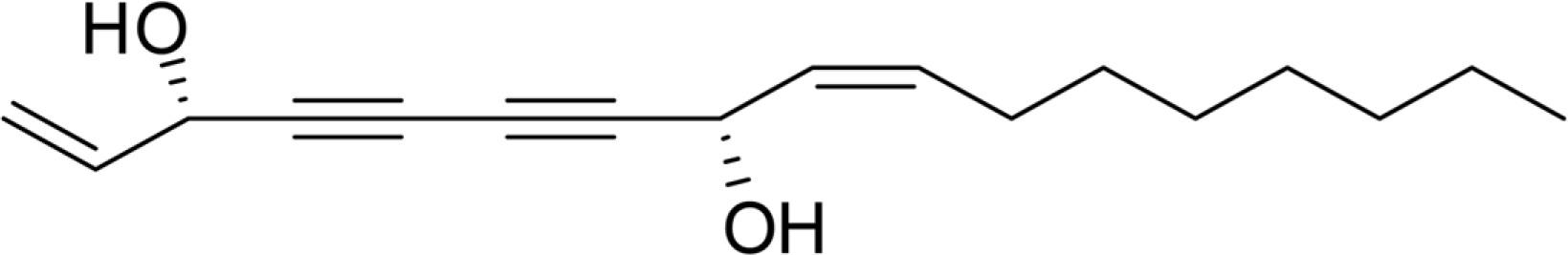
Angelica koreana is an important medicinal plant for some locals in East Asia including Korea. A few reports have shown the efficacy of its phytochemical constituents. We have isolated and purified one compound falcarindiol (FAL) from the methanolic extract of A. koreana roots. At concentrations from to 1 µM to 25 µM, the FAL isolated from the roots of A. koreana exerted no significant cytotoxicity and down-regulated LPS-stimulated pro-inflammatory cytokine IL-8 in colon epithelial cells, while up-regulating anti-inflammatory cytokine IL-10. In addition, the FAL decreased the expression of LPS-induced inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 protein by Western blot analysis. Colon epithelial cells play pivotal roles in regulating the colon immune system and thus FAL is expected to be candidate agent as therapeutic potential for the treatment of inflammatory bowel disease (IBD) by modulating LPS-induced inflammation in colon epithelial cells.
Go to : 
References
(1). Dinarello C. A.Chest. 1997. 112:,. 321S–329S.
(2). Mariani F.., Sena P.., Roncucci L.World J. Gastroenterol. 2014. 20:9716–9731.
(3). Kang S. S.., Noh S. Y.., Park O. J.., Yun C. H.., Han S. H.Cytokine. 2015. 75:174–180.
(4). Bogdan C.., Paik J.., Vodovotz Y.., Nathan C. J.Biol. Chem. 1992. 267:23301–23308.
(5). Kang K. H.., Kong C. S.., Seo Y.., Kim M. M.., Kim S. K.Food Chem. Toxicol. 2009. 47:2129–2134.
(6). Sarker S. D.., Nahar L.Curr. Med. Chem. 2004. 11:1479–1500.
(7). Kim H. S.., Lee Y. J.., Lee H. K.., Kim J. S.., Park Y.., Kang J. S.., Hwang B. Y.., Hong J. T.., Kim Y.., Han S. B.Food Chem. Toxicol. 2013. 59:26–33.
(8). Lee J. W.., Yun C. Y.., Roh E.., Lee C.., Jin Q.., Bang K. K.., Jung S. H.., Lee D.., Lee M.K.; Kim, Y.; Hwang, B.Y. Bioorg. Med. Chem. Lett. 2012. 22:2927–2931.
(9). Seo E. K.., Kim K. H.., Kim M. K.., Cho M. H.., Choi E. W.., Kim K. N.., Mar W.Planta Med. 2002. 68:162–163.
(10). Oh M. S.., Yang J. Y.., Lee H. S. J.Agric. Food Chem. 2012. 60:3606–3611.
(11). Yoshikawa M.., Nishida N.., Ninomiya K.., Ohgushi T.., Kubo M.., Morikawa T.., Matsuda H.Bioorg. Med. Chem. 2006. 14:456–463.
(12). Metzger B. T.., Barnes D. M.., Reed J. D. J.Agric. Food Chem. 2008. 56:3554–3560.
(13). Daig R.., Andus T.., Aschenbrenner E.., Falk W.., Schölmerich J.., Gross V.Gut. 1996. 38:216–222.
(14). Subramanian S.., Rhodes J. M.., Hart C. A.., Tam B.., Roberts C. L.., Smith S. L.., Corkill J. L.., Winstanley C.., Virji M.., Campbell B. J.Inflamm. Bowel Dis. 2008. 14:162–175.

(15). Kim J.., Kim J. S.., Park E.Food Chem. Toxicol. 2013. 62:199–204.
(16). Kankuri E.., Asmawi M. Z.., Korpela R.., Vapaatalo H.., Moilanen E.Inflammation. 1999. 23:141–152.

(17). Takahashi A.., Yamamoto N.., Murakami A.Life Sci. 2011. 89:337–342.
(18). Zschocke S.., Lehner M.., Bauer R.Planta Med. 1997. 63:203–206.
(19). Deng S.., Wang Y.., Inui T.., Chen S. N.., Farnsworth N. R.., Cho S.., Franzblau S. G.., Pauli G. F.Phytother. Res. 2008. 22:878–882.
(20). Sun S.., Du G. J.., Qi L. W.., Williams S.., Wang C. Z.., Yuan C. S. J.Ethnopharmacol. 2010. 132:280–285.
(21). Lee S. K.., Kim T. I.., Kim Y. K.., Choi C. H.., Yang K. M.., Chae B.., Kim W. H.Biochem. Biophys. Res. Commun. 2005. 337:457–463.
(22). Han Y.., Ma T. M.., Lu M. L.., Ren L.., Ma X. D.., Bai Z. H.World J. Gastroenterol. 2014. 20:11297–11304.
(23). Nahidi L.., Leach S. T.., Mitchell H. M.., Kaakoush N. O.., Lemberg D. A.., Munday J. S.., Huinao K.., Day A. S.Biomed. Res. Int. 2013. 2013:909613.
(24). Mitsui S.., Torii K.., Fukui H.., Tsujimura K.., Maeda A.., Nose M.., Nagatsu A.., Mizukami H.., Morita A. J.Pharmacol. Exp. Ther. 2010. 333:954–960.
(25). Jeong J. B.., Shin Y. K.., Lee S. H.Food Chem. Toxicol. 2013. 55:229–233.
Go to : 
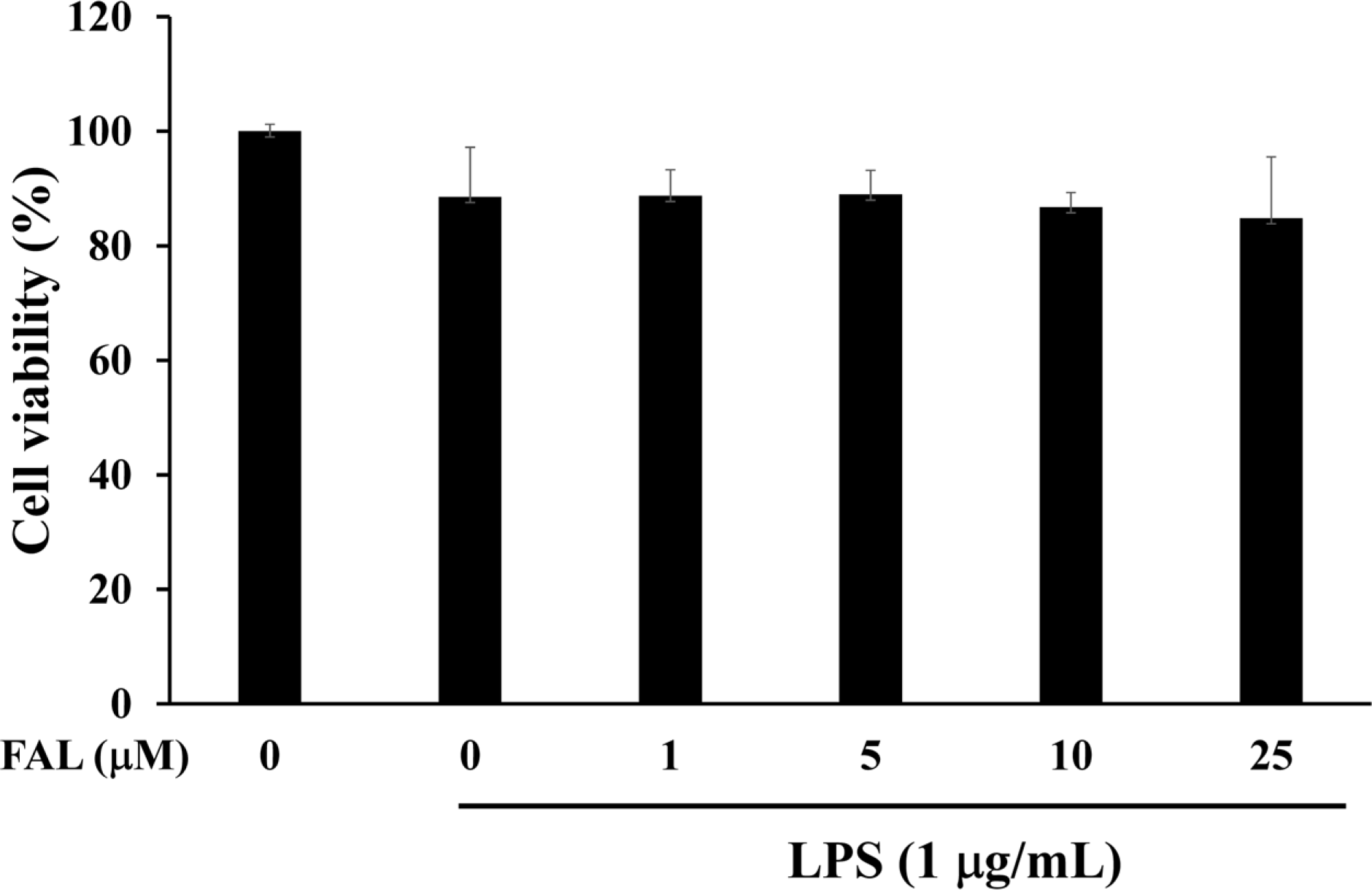 | Fig. 2.Effects of FAL on the cell viability of HT-29 colon epithelial cells. The cells were preincubated in the presence of the indicated concentrations of FAL for 1 h and then treated with LPS (1 µg/mL) for 24 h. Cell viability was assessed using the CCK-8 kit. The results are presented as the mean ± SD of three individual experiments. |
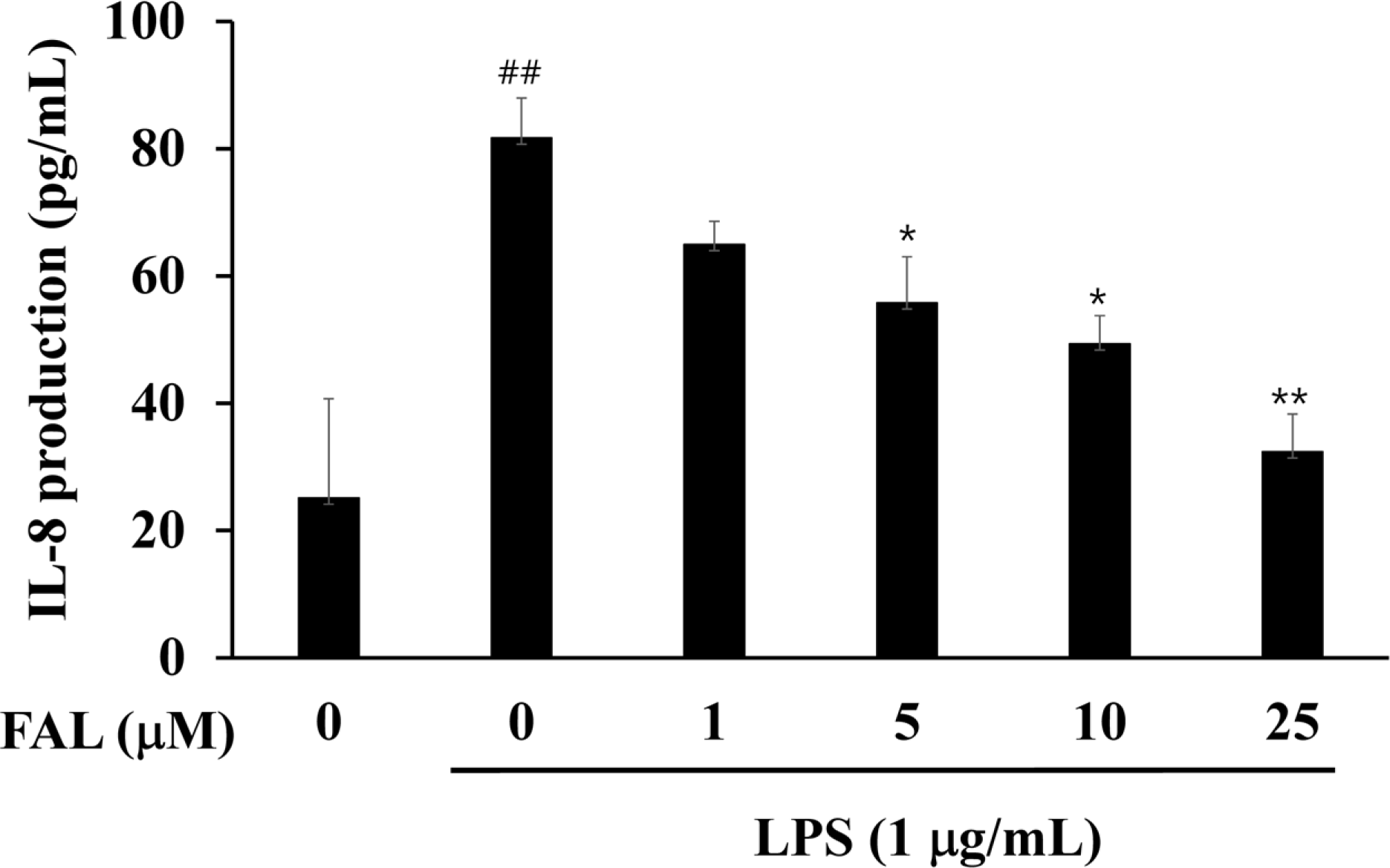 | Fig. 3.Effects of FAL on IL-8 production in LPS-stimulated colon epithelial cells. The cells were preincubated for 1h with FAL, then incubated with LPS for 12 h. IL-8 levels were then determined by ELISA (mean ± SD, n=3). ## p <0.01, compared with untreated control; ∗p < 0.05 and ∗∗ p < 0.01, compared with LPS-treated control. |
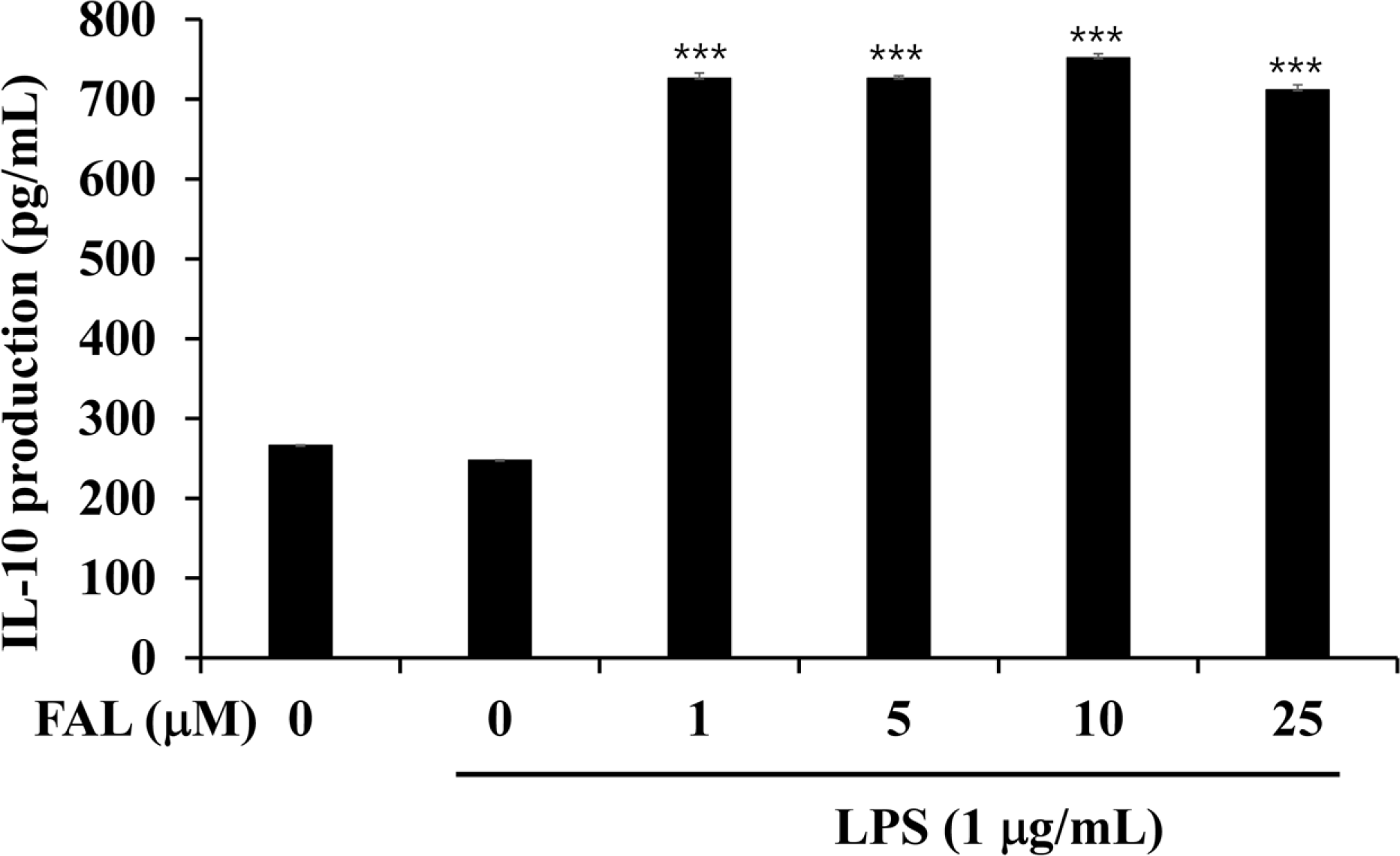 | Fig. 4.Effects of FAL on IL-10 production in LPS-stimulated colon epithelial cells. The cells were preincubated for 1 h with FAL, then incubated with LPS for 12 h. IL-10 levels were then determined by ELISA (mean ± SD, n=3). ∗∗∗ p <0.001, compared with LPS-treated control. |
 | Fig. 5.Effects of FAL on the expression of LPS-induced iNOS and COX-2 protein in HT-29 colon epithelial cells. Cells were pretreated with FAL at the indicated concentrations for 1 h and then treated with LPS (1 µg/mL) for 24 h. Cell lysates were prepared and the iNOS, COX-2 and actin protein levels were determined by western blotting. The relative intensity of iNOS and COX-2 to actin bands was measured by densitometry. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download