Abstract
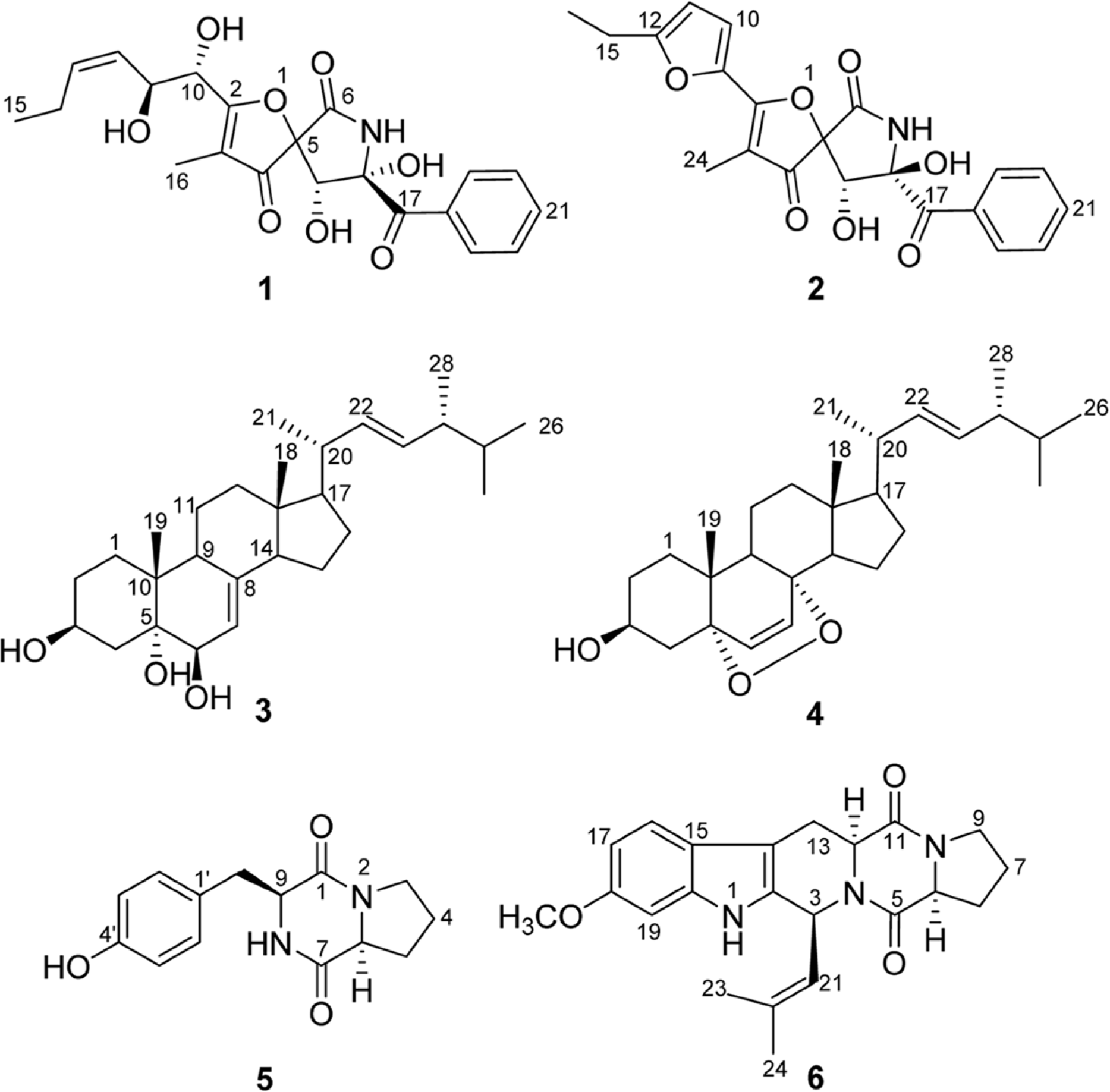
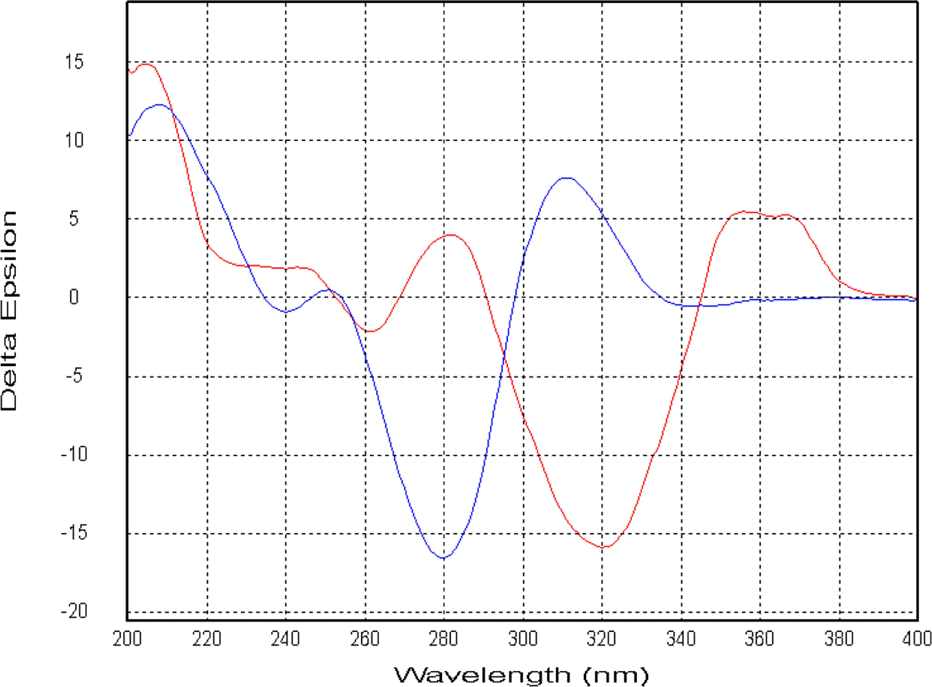
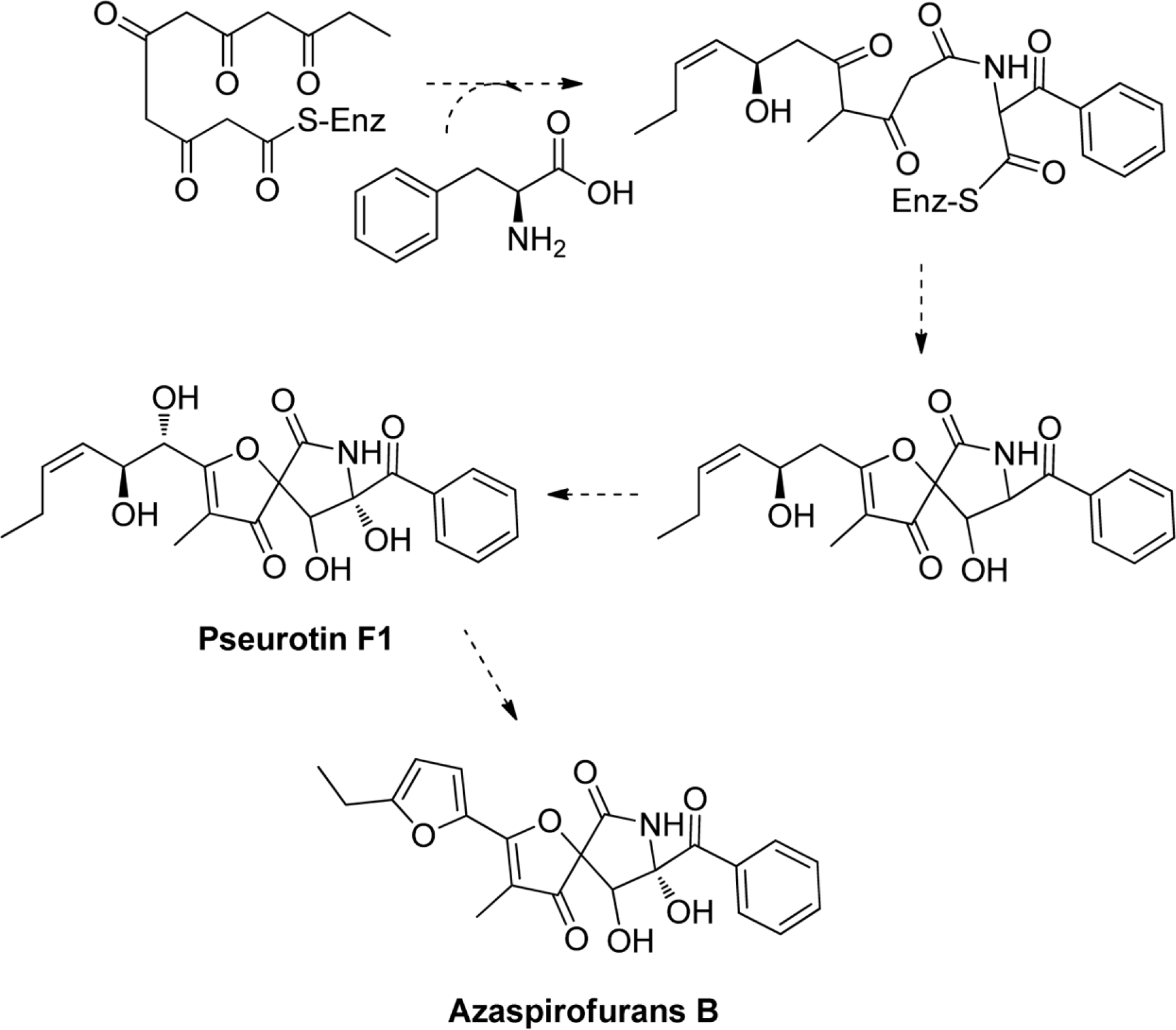
Six compounds were isolated from the secondary metabolites of the jellyfish-derived fungus Aspergillus fumigates, whose structures were identified by chemical methods and spectroscopic analysis as pseurotin F1 (1), azaspirofurans B (2), (22 E, 24 R)-24-methyl-5α-cholesta-7,22-diene-3β,5,6β-triol (3), 5α,8α-epidioxyergosta-6,22-dien-3β-o1 (4), cyclo-(L-Pro-L-Tyr) (5), fumitremorgin C (6). The compounds 1–5 were isolated from the fungus Aspergillus fumigates for the first time. The isolated compounds (1–6) were evaluated for antibiotic activity and cytotoxicity against six bacterial strains and ten human tumor cell lines, respectively.
REFERENCES
(1). Mille-Lindblom C., Fischer H., Tranvik L. J.OIKOS. 2006; 113:233–242.
(2). Zhang H. W., Song Y. C., Tan R. X.Nat. Prod. Rep. 2006; 23:753–771.
(3). Blunt J. W., Copp B. R., Keyzers, R. A. Munro M. H., Prinsep M. R.Nat. Prod. Rep. 2014; 31:160–258.
(4). Uye S.Plankton Benthos Res. 2008; 3:125–131.
(5). Wink J., Grabley S., Gareis M., Thiericke R., Kirsch R.European Patent Application. 1993; EP0546474A1.
(6). Frisvad J. C., Rank C., Nielsen K. F.Med. Mycol. 2009; 47:S53–S71.
(7). Wenke J., Anke H., Sterner O.Biosci. Biotech. Biochem. 1993; 57:961–964.
(8). Ren H., Liu R., Chen, L. Zhu T., Zhu W. M., Gu Q. Q.Arch. Pharm. Res. 2010; 33:499–502.
(9). Mohr P., Tamm C.Tetrahedron. 1981; 37:201–212.
(9). Mohr P., Tamm C.Tetrahedron Lett. 1987; 28:391–394.
(10). Tsunematsu Y., Fukutomi M., Saruwatari, T. Noguchi H., Hotta K., Tang Y., Watanabe K.Angew. Chem. Int. Ed. Engl. 2014; 53:8475–8479.
(11). Vincenzo P., Donato S. J.Nat. Prod. 1987; 50:915–920.
(12). Wang X., Wang H., Liu T., Xin Z.Appl. Microbiol. Biotechnol. 2014; 98:4875–4885.
(13). Ioannou E., Abdel-Razik A. F., Zervou M., Christofidis D., Alexi X., Vagias C., Alexis M. N., Roussis V.Steroids. 2009; 74:73–80.
(14). Gauvin A., Smadja J., Aknin M. Can. J.Chem. 2000; 78:986–992.
(15). Bu M., Yang B. B., Hu L.Med. Chem. 2014; 4:709–716.
(16). Jayatilake G. S., Thornton M. P., Leonard A. C. J.Nat. Prod. 1996; 59:293–296.
(17). Cimmino A., Puopolo G., Perazzolli M., Andolfi A., Melck D., Pertot I., Evidente A.Chem. Heterocycl. Compds. 2014; 50:290–295.
(18). Borthwick A. D.Chem. Rev. 2012; 112:3641–3716.
(19). Zhang D., Noviendri D., Nursid M., Yang X., Son B. W.Nat. Prod. Sci. 2007; 13:251–254.
(20). Puopolo G., Cimmino A., Palmieri, M. C. Giovannini O., Evidente A., Pertot I. J.Appl. Microbiol. 2014; 117:1168–1180.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download