Abstract
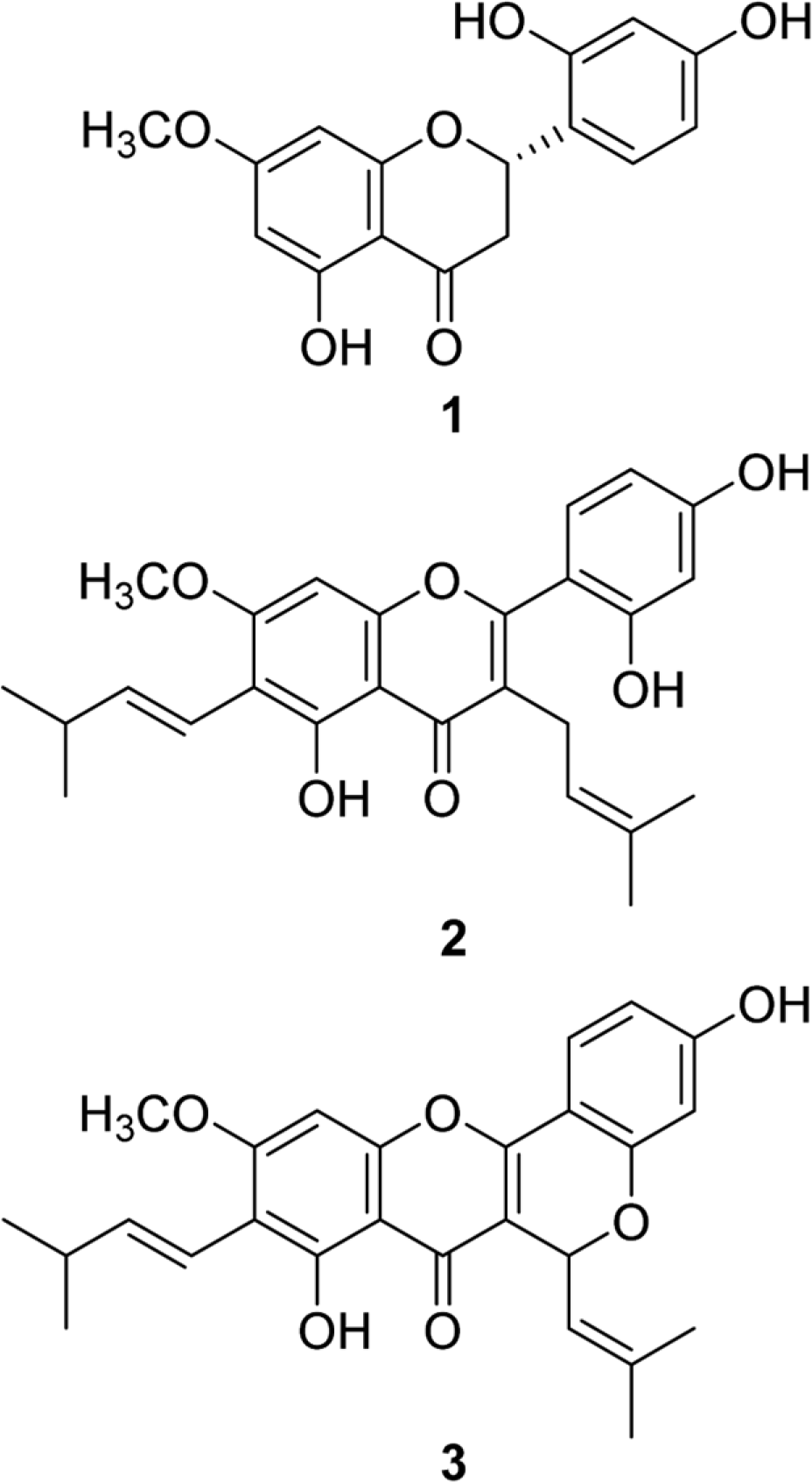
A reversed-phase high-performance liquid chromatographic method is described for the simultaneous determination of three antibacterial flavonoids, artocarpanone, artocarpin, and cycloartocarpin in ethyl acetate extracts from Artocarpus heterophyllus heartwoods. Separation was achieved using a TSK-gel ODS-80Tm column (5 µm, 4.6 × 150 mm) at 25oC with a gradient elution system of methanol and water as follows: 0-8 min,60:40; 8-27 min, 80:20; 27-35 min, 60:40, v/v, at a flow rate of 1 mL/min, and a quantitative UV detection at 285 nm. The method was validated by measuring the key parameters, including specificity, linearity, sensitivity, accuracy, repeatability and reproducibility. A high degree of specificity and sensitivity was achieved. The calibration curves for all three flavonoids showed good linearity with a coefficient of determinations (R2) of ≥ 0.9995. The recoveries of the method were from 98-104%, with good reproducibility and repeatability (RSD values of less than 2%) were also achieved. Ethyl acetate was the best solvent for extraction of these three flavonoids using the heat reflux conditions for 1 h. This optimized sample preparation and HPLC method can be practically used for a routine standardization process of the extracts from the A. heterophyllus heartwoods.
Go to : 
REFERENCES
(1). Salguero C.P.; A Thai herbal traditional recipes for health and harmony; Findhorn press; Scotland. 2003; 119.
(2). Arung E. T., Yoshikawa K., Shimizu K., Kondo R.Fitoterapia. 2010; 81:120–123.
(3). Sato M., Fujiwara S., Tsuchiya H., Fujii T., Iinuma M., Tosa H., Ohkawa Y. J.Ethnopharmacol. 1996; 54:171–176.
(4). Ko F. N., Cheng Z. J., Lin C. N., Teng C. M.Free Radic. Biol. Med. 1998; 25:160–168.
(5). Zheng Z. P., Chen S., Wang S., Cheng K. W., Wu J. J. J.Agric. Food Chem. 2009; 57:6649–6655.
(6). Septama A. W., Panichayupakaranant P.Pharm. Biol. 2015; 53:1608–1613.
(7). Wei B. L., Weng J. R., Chiu P. H., Hung C. F., Wang J. P., Lin C. N. J.Agric. Food Chem. 2005; 53:3867–3871.
(8). Dej-Adisai S., Meechai I., Puripattanavong J., Kummee S.Arch. Pharm. Res. 2014; 37:473–483.
(9). Arung E. T., Wicaksono B. D., Handoko Y. A., Kusuma I. W., Shimizu K., Yulia D., Sandra F. J.Nat. Med. 2010; 62:423–429.
(10). Kirchmair J., Rollinger J. M., Liedl K. R., Seidel N., Krumbholz A., Schmidtke M.Future Med. Chem. 2011; 3:437–450.
(11). Chung M. I., Weng J. R., Wang J. P., Teng C. M., Lin C. N.Planta Med. 2002; 68:25–29.
(12). Shabir G. A. J.Chromatogr. A. 2003; 987:57–66.
(13). ICH. Guideline Q2(R1)-Validation of Analytical Procedure: Text and methodology. ICH;Geneva: 2005. p. 1–13.
(14). Zheng Z. P., Chen S., Wang S., Wang X. C., Cheng K. W., Wu J. J., Yang D., Wang M. J.Agric. Food Chem. 2009; 57:6649–6655.
Go to : 
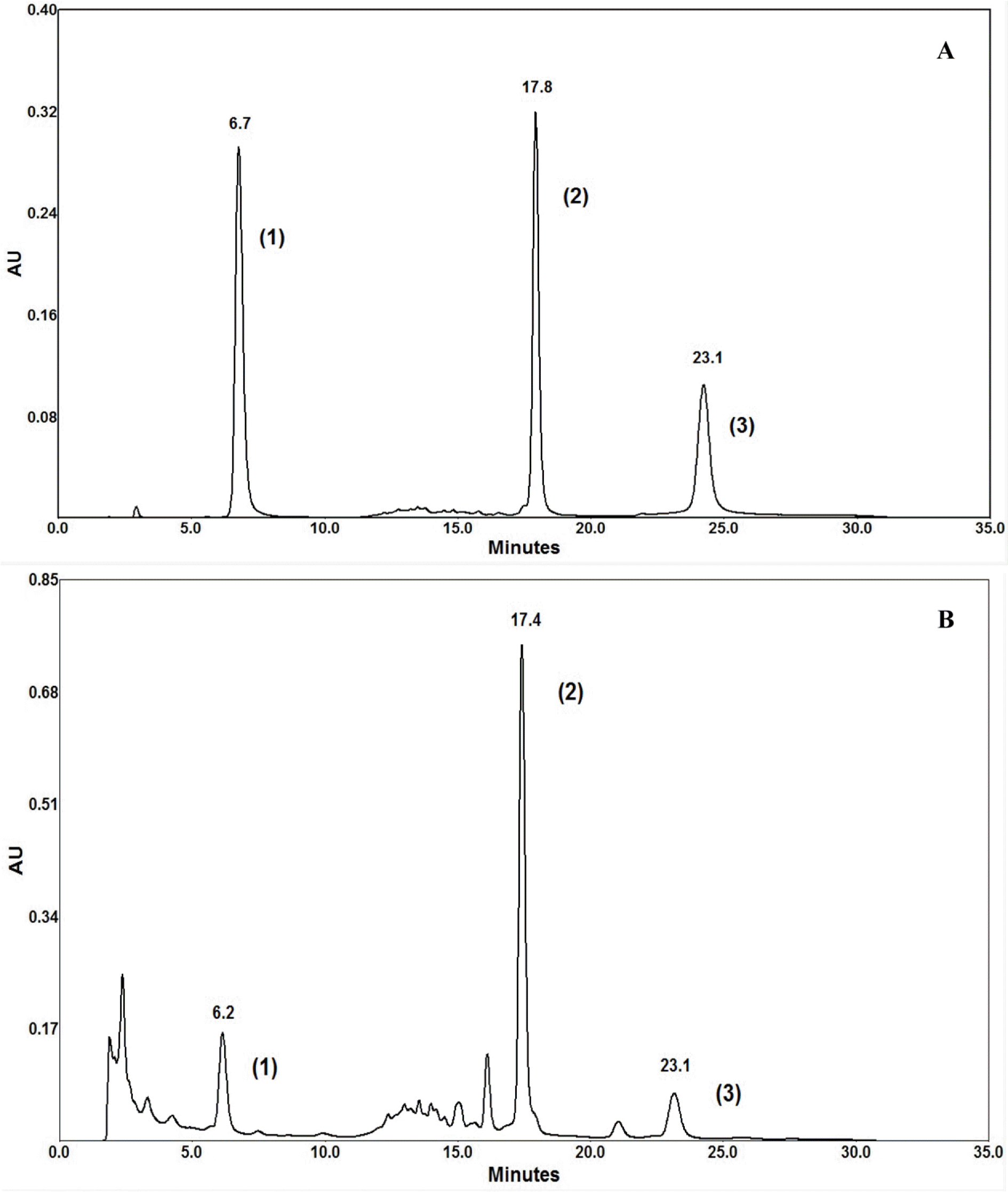 | Fig. 2.HPLC chromatograms of (A) authentic compounds an (B) heartwood extract of A. heterophyllus. (1: Artocarpanone, 2 Artocarpin, 3: Cycloartocarpin). |
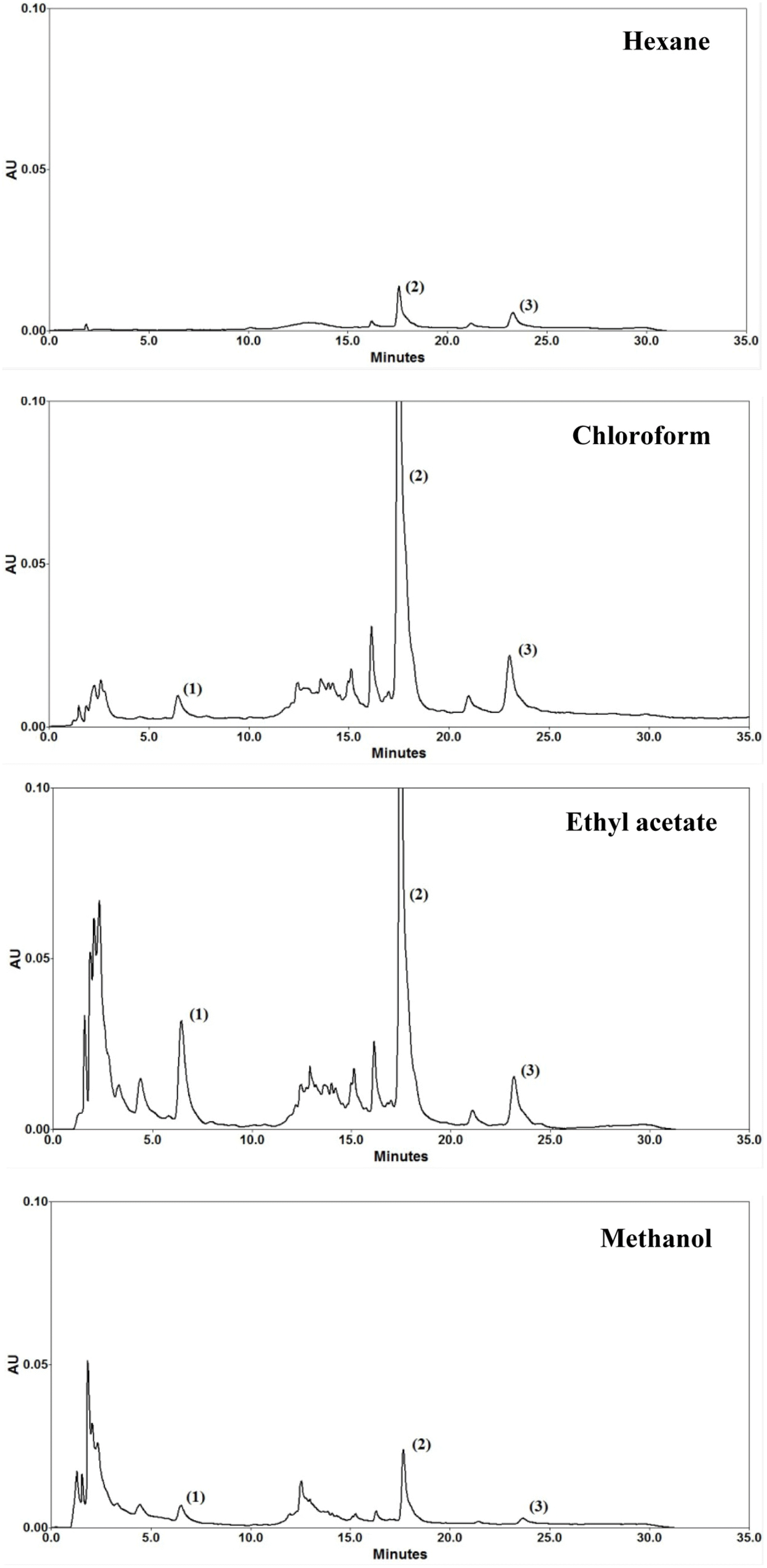 | Fig. 3.HPLC chromatograms of hexane, chloroform, ethyl acetate, and methanol extracts from of A. heterophyllus heartwoods. |
Table 1.
HPLC calibration data for artocarpanone, artocarpin, and cycloartocarpin
| Compounds | Linear range (µg/mL) | tR (min) | Equationa | Linearity (R2) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|
| Artocarpanone | 6.25–100 | 6.7 | Y = 52290 X − 35882 | 0.9997 | 0.06 | 0.2 |
| Artocarpin | 6.25–100 | 17.8 | Y = 47287 X − 44018 | 0.9998 | 0.04 | 0.2 |
| Cycloartocarpin | 6.25–100 | 23.1 | Y = 25630 X − 37434 | 0.9995 | 0.2 | 0.4 |
Table 2.
Recovery data for the three flavonoids spiked into A. heterophyllus heartwood extracts
Table 3.
Intraday and interday precision data for the quantitative determination of the three flavonoids
| Compounds | RSD (%) | |
|---|---|---|
| intra-day (n = 6) | interday (n = 3) | |
| Artocarpanone | 0.76 | 1.23 |
| Artocarpin | 0.78 | 1.49 |
| Cycloartocarpin | 0.64 | 1.27 |
Table 4.
Content of the three flavonoids in different solvents for extracting A. heterophyllus heartwood
| Solvents | Yield of dried extracts (% w/w; mean ± SD) | Content (%w/w of dried extract; mean ± SD) | ||
|---|---|---|---|---|
| Artocarpanone | Artocarpin | Cycloartocarpin | ||
| Hexane | 2.2 ± 0.07∗ | n.a. | 52.84 ± 0.16∗ | 2.62 ± 0.02∗ |
| Chloroform | 5.4 ± 0.15∗ | 1.03 ± 0.03∗ | 21.72 ± 0.57 | 5.51 ± 0.12 |
| Ethyl acetate | 7.2 ± 0.20 | 4.95 ± 0.01 | 21.84 ± 0.60 | 5.75 ± 0.20 |
| Methanol | 25.6 ± 1.47∗ | 1.09 ± 0.05∗ | 53.01 ± 0.10∗ | 1.09 ± 0.03∗ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download