Abstract
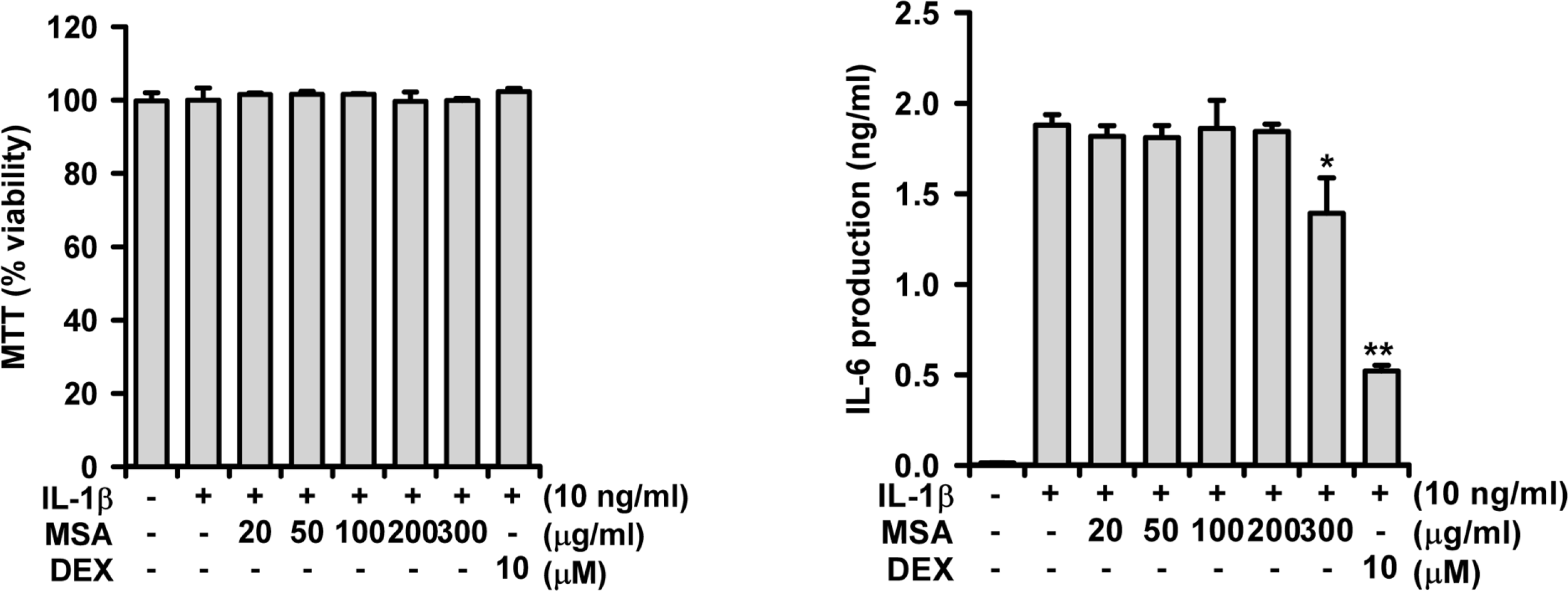
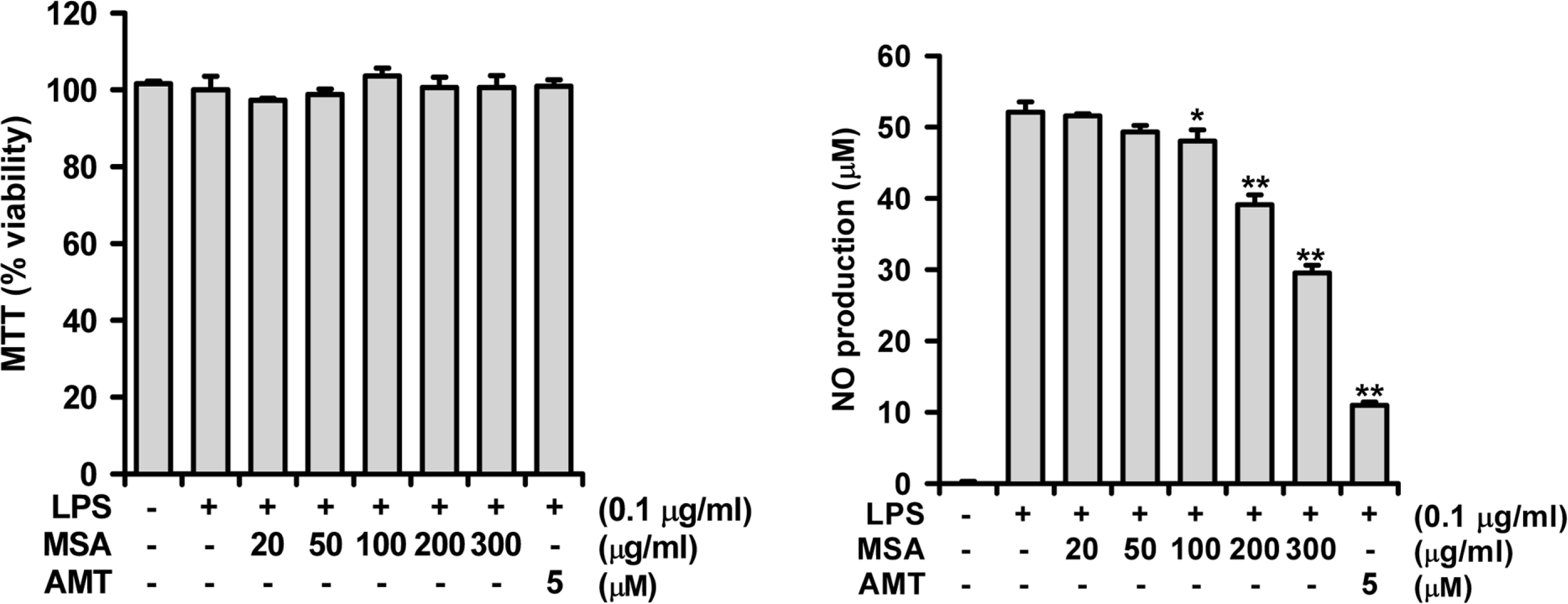
A phytoformula containing the root barks of Morus alba, the fructus of Schizandra sinensis and the roots of Asparagus cochinchinensis (MSA) was prepared as a potential new herbal remedy, and its therapeutic potential for alleviating inflammatory lung conditions was examined. For in vivo evaluation, an animal model of lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice was used. With oral administration of 6–60 mg/kg, MSA potently and dose-dependently inhibited bronchitis-like symptoms in acute lung injury induced by intranasal treatment of LPS as judged by the number of cells in the bronchoalveolar lavage fluid (BALF) and histological observation. The inhibitory potency was comparable with that of dexamethasone. For in vitro assay, the effects on the production of proinflammatory molecules in lung epithelial cells and alveolar macrophages were examined. Although MSA inhibited IL-6 production in IL-1β-treated lung epithelial cells (A549) only at a high concentration (300 μg/ml), the formula strongly and concentration-dependently inhibited NO production in LPS-treated alveolar macrophages (MH-S) at 20–300 μ g/ml. Based on all of these findings, the new phytoformula MSA is suggested to have the potential to control inflammatory lung diseases including bronchitis, at least in part, by inhibiting inducible nitric oxide synthase-catalyzed NO production.
Go to : 
REFERENCES
(1). Jeffery P. K. Am. J.Respir. Crit. Care Med. 2001; 164:S28–S38.
(2). Barnes P. J.Clin. Chest Med. 2014; 35:71–86.
(3). Bae K.The medicinal plants of Korea. Kyo-Hak Pub. Co.;Korea: 2000. p. 73.
(4). Hong C. H., Hur S. K., Oh O. J., Kim S. S., Nam K. A, Lee S. K. J.Ethnopharmacol. 2002; 83:153–159.
(5). Chao W. W., Kuo Y. H., Li W. C., Lin B. F. J.Ethnopharmacol. 2009; 122:68–75.
(6). Cheon B. S., Kim Y. H., Son K. S., Chang H. W., Kang S. S., Kim H. P.Planta Med. 2000; 66:596–600.
(7). Yang Z. G., Matsuzaki K., Takamatsu S., Kitanaka S.Molecules. 2011; 16:6010–6022.
(8). Lim H. J., Jin H. G., Woo E. R., Lee S. K., Kim H. P. J.Ethnopharmacol. 2013; 149:169–175.
(9). Bae K.The medicinal plants of Korea. Kyo-Hak Pub. Co.;Korea: 2000. p. 116.
(10). Lim H., Son K. H., Bae K. H., Hung T. M., Kim Y. S., Kim H. P.Phytother. Res. 2009; 23:1489–1492.
(11). Kim H., Ahn Y. T., Kim Y. S., Cho S. I., An W. G.Pharmacogn. Mag. 2014; 10:S80–S85.
(12). Bae H., Kim R., Kim Y., Lee E., Kim H. J., Jang Y. P., Jung S. K., Kim J. J.Ethnopharmacol. 2012; 142:41–47.
(13). Lee H. J., Park J. S., Yoon Y. P., Shin Y. J., Lee S. K., Kim Y. S., Hong J. H., Son K. H., Lee C. J.Phytomedicine. 2015; 22:568–572.
(14). Lee J. H., Lim H. J., Lee C. W., Son K. H., Son J. K., Lee S. K., Kim H. P.Evid. Based Complement Alternat. Med. 2015; DOI: doi:10.1155/2015/640846.
(15). Mosmann T. J.Immunol. Methods. 1983; 65:55–63.
(16). Ko H. J., Jin J. H., Kwon O. S., Kim J. T., Son K. H., Kim H. P.Biomol. Ther. 2011; 19:324–330.
(17). Nomura T.Yakugaku Zasshi. 2001; 121:535–556.
(18). Zhu L., Li B., Liu X., Huang G., Meng X.Food Chem. 2015; 186:146–152.
(19). Kim S. Y., Son K. H., Chang H. W., Kang S. S., Kim H. P.Arch. Pharm. Res. 1999; 22:313–316.
(20). Guo R., Pittler M. H., Ernst E.Eur. Respir. J. 2006; 28:330–338.
(21). Sharma M., Arnason J. T., Burt A., Hudson J. B.Phytother. Res. 2006; 20:147–152.
(22). Agbabiaka T. B., Guo R., Ernst E.Phytomedicine. 2008; 15:378–385.
(23). Matthys H., Funk P.Planta Med. 2008; 74:686–692.
Go to : 
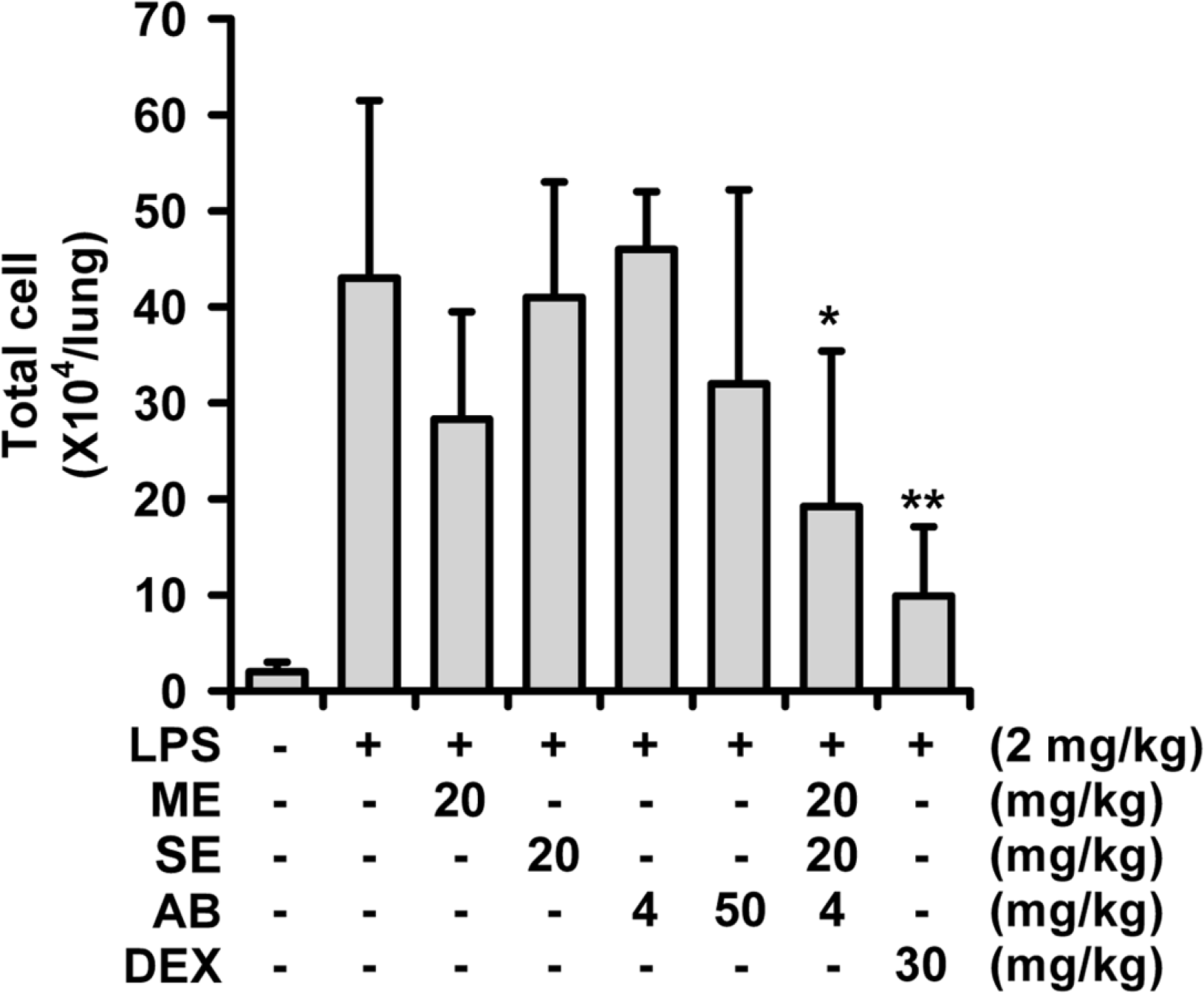 | Fig. 1.Potentiation of MSA in inhibiting LPS-induced acute lung injury in mice.
The number of total cells in the BALF is presented here, and BALF was obtained 16 h after LPS-treatment in mice. ME: 70% ethanol extract of M. alba, SE, 70% ethanol extract of S. chinensis, AB: n-butanol fraction of A. cochinchinensis, DEX: dexamethasone, ∗P < 0.05, ∗∗P < 0.01, Significantly different from the LPS-treated control group (n = 7).
|
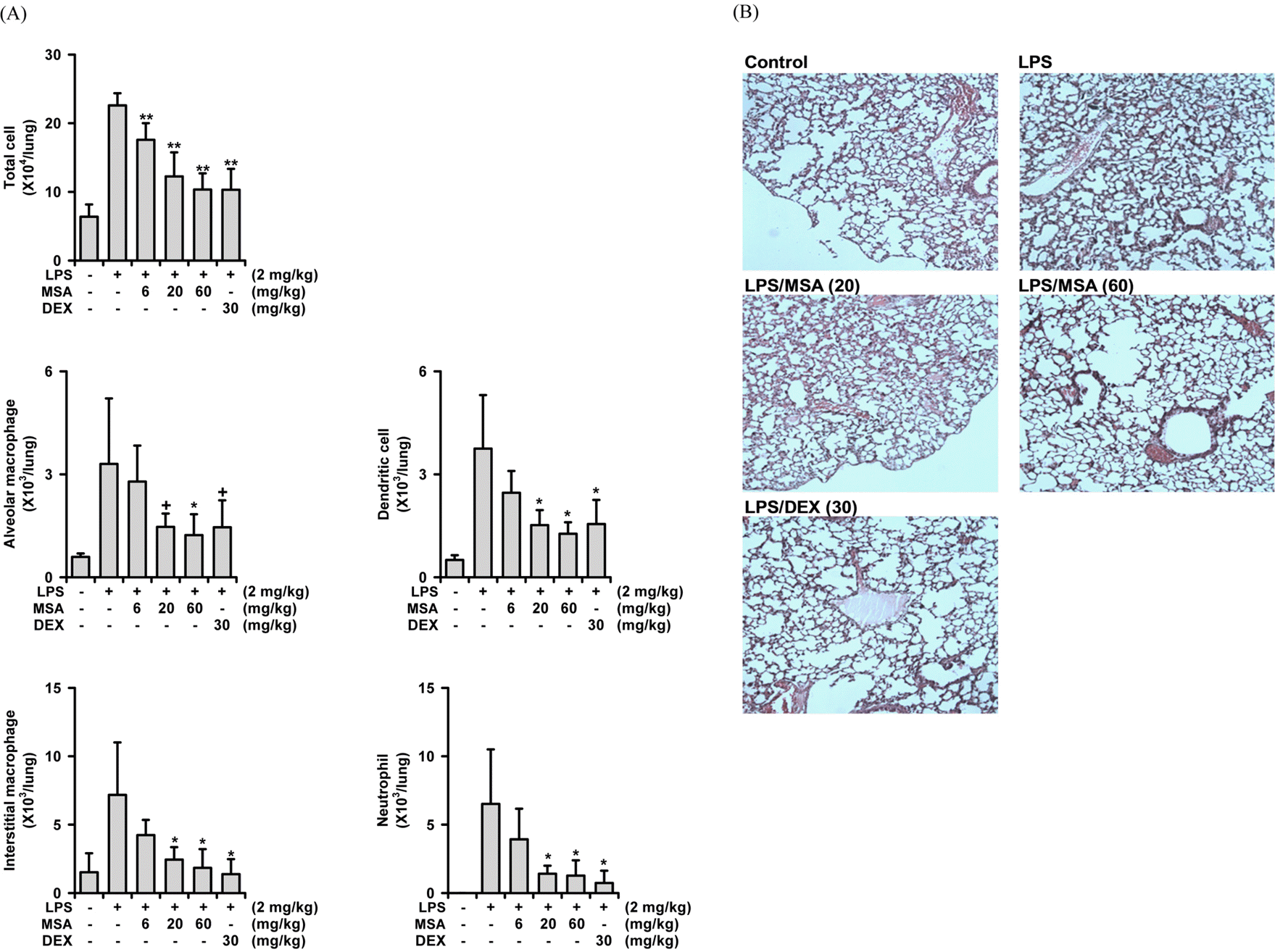 | Fig. 2.Dose-dependent inhibition of MSA against LPS-induced acute lung injury in mice.
Mice were sacrificed 16 h after the LPS challenge. From the BALF, the numbers of cells were counted. (A) Effects on the cell numbers in the BALF. Total cell numbers were counted using a haemocytometer. FACS was used to differentiate between each type of inflammation-related cell. DEX: dexamethasone,+P < 0.1, ∗P < 0.05, ∗∗P < 0.01, Significantly different from the LPS-treated group (n = 7). (B) Histological observation of lung tissues (H&E staining). Presented here is the result of one sample from two sets stained (× 100).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download