Abstract
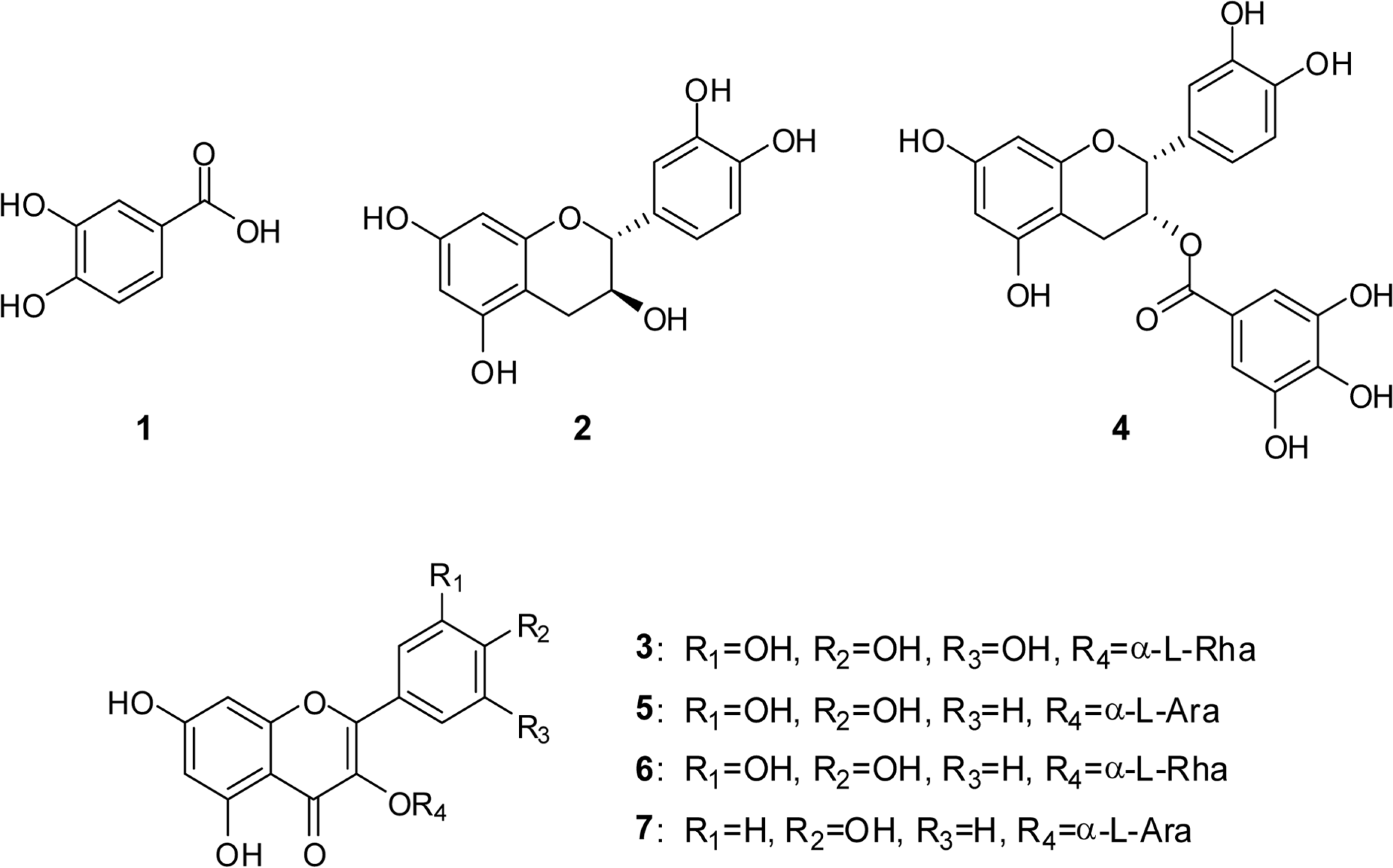
A method for simultaneously identifying antioxidative compounds was developed using time-based LC-MS coupled with DPPH assay regardless of the time consuming process. The methanolic extract of Polygonum aviculare (Polygonaceae) showed significant DPPH radical scavenging activity. Time-based DPPH assay for simultaneous identification of active compounds from the extracts of P. aviculare was used. Major peaks of ethyl acetate fraction of P. aviculare showed high DPPH radical scavenging activity. A simple phenolic compound (1) and six flavonoids (2-7) were isolated from the ethyl acetate fraction of P. aviculare by silica gel and sephadex LH-20 column chromatography. The structures of seven compounds were determined to be protocatechuic acid (1), catechin (2), myricitrin (3), epicatechin-3-O-gallate (4), avicularin (5), quercitrin (6), and juglanin (7) based on the analysis of the1H-NMR,13C-NMR and ESI-MS data. All compounds exhibited significant antioxidant activity on DPPH assay and active compounds were well correlated with predicted one.
REFERENCES
(1). Qu F. N., Qi L. W., Wei Y. J., Wen X. D., Yi L., Luo H. W., Li P.Biol. Pharm. Bull. 2008; 31:501–506.
(2). Nuengchamnong N., Krittasilp K., Ingkaninan K.Food Chem. 2011; 127:1287–1293.
(3). Nuengchamnong N., Krittasilp K., Ingkaninan K.Food Chem. 2009; 117:750–756.
(4). Nugroho A., Kim E. J., Choi J. S., Park H. J. J.Pharm. Biomed. Anal. 2014; 89:93–98.
(5). Robu T., Robu B., Robu S.Environ. Eng. Manag. J. 2008; 7:579–588.
(6). Granica S., Piwowarski J. P., Pop³awska M., Jakubowska M. J.Pharm. Biomed. Anal. 2013; 72:216–222.
(7). Park S. H., Sung Y. Y., Nho K. J., Kim H. K. J.Ethnopharmacol. 2014; 151:1109–1115.
(8). Sung Y. Y., Yoon T., Yang W. K., Kim S. J., Kim D. S., Kim H. K.Evid. Based Complement Alternat. Med. 2013; 2013:626397.
(9). Hsu C. Y.Biol. Res. 2006; 39:281–288.
(10). Granica S., Czerwi ska M. E., Zy y ska-Granica B., Kiss A. K.Fitoterapia. 2013; 91:180–188.
(11). Nguyen P. H., Zhao B. T., Lee J. H., Kim Y. H., Min B. S., Woo M. H.Bull. Korean Chem. Soc. 2014; 35:1763–1768.
(12). Seto R., Nakamura H., Nanjo F, Hara Y.Biosci. Biotech. Biochem. 1997; 61:1434–1439.
(13). Aderogba M. A, Ndhlala A. R, Rengasamy K. R, Van Staden J.Molecules. 2013; 18:12633–12644.
(14). Kim H. J., Woo E. R., Park H. K. J.Nat. Prod. 1994; 57:581–586.
(15). Kwon D. J., Bae Y. S.Chem. Nat. Compd. 2013; 49:131–132.
(16). Park B. J., Matsuta T., Kanazawa T., Park C. H., Chang K. J., Onjo M.Chem. Nat. Compd. 2012; 48:477–479.
(17). Magiera S., Baranowska I., Lautenszleger A. J.Pharm. Biomed. Anal. 2015; 102:468–475.
(18). de Beck P. O., Duoux M. G., Cartier G., Mariotte A. M.Phytochemistry. 1998; 47:1171–1173.
(19). Sroka Z., Cisowski W.Food Chem. Toxicol. 2003; 41:753–758.
Fig. 1.
HPLC chromatogram (254 nm) and time-dependent DPPH assay results of EtOAc fraction from P. aviculare (A), expansion of the HPLC chromatogram between 11–16 min (B), UV spectrum of each peaks (C).
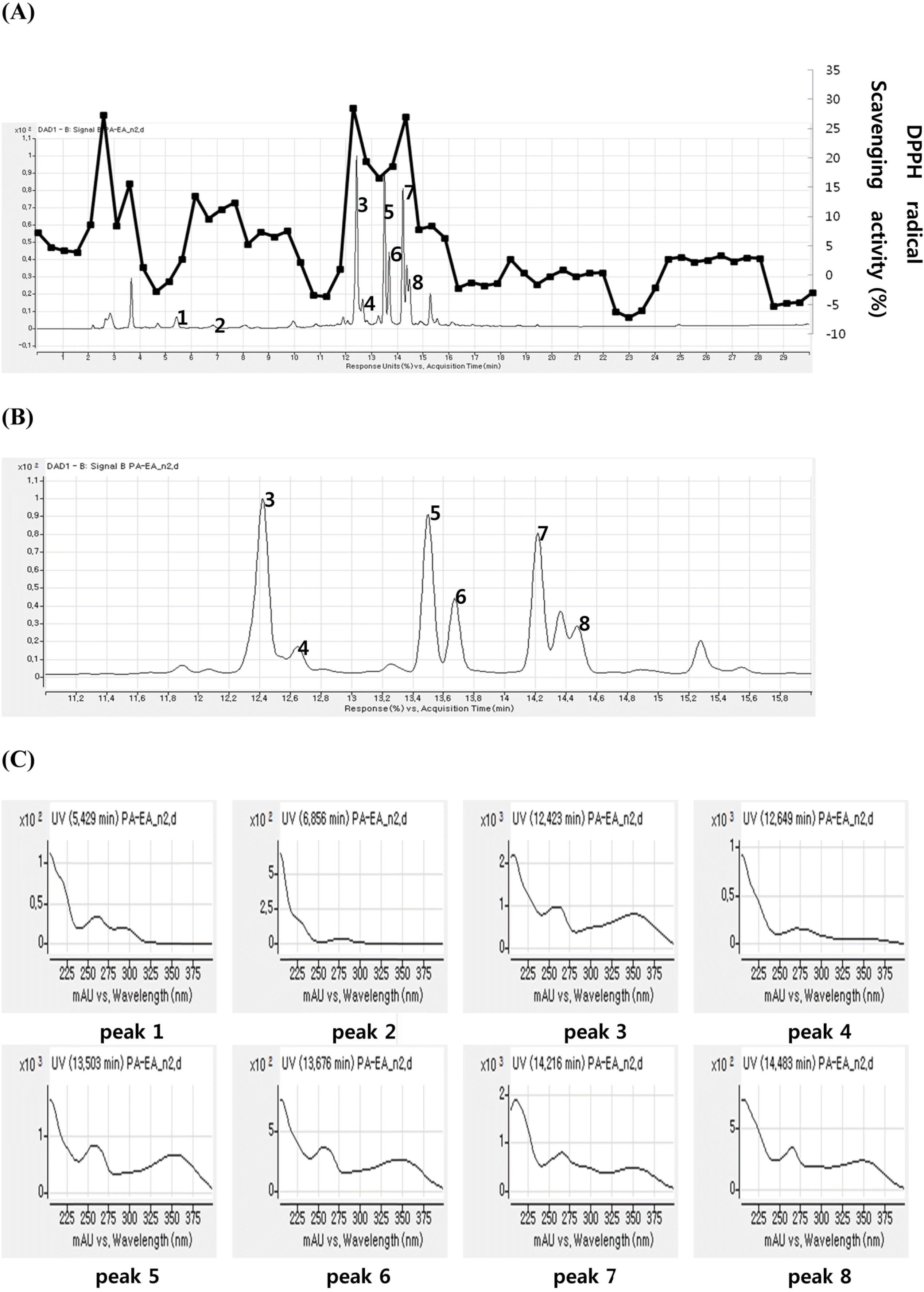
Table 1.
DPPH radical scavenging activity of the extract and fractions from P. aviculare
Table 2.
LC-MS analysis of each peak in EtOAc fractions from P. aviculare




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download