Abstract
The study is a pioneering effort to determine the mineral, nutritional, and phytochemical composition and phenolic content and to determine the free radical scavenging activity of Gigantochloa levis (Blanco) Merr, a native bamboo species (locally known as “bolo”) in the Philippines. Proximate analysis showed that air-dried G. levis leaves contain 15.8% ash, 22.6% crude protein, 1.2% crude fat, 29.3% crude fiber, and 19.7% total sugar. Phytochemical tests indicated the presence of diterpenes, triterpenes, saponins, phenols, tannins, and flavonoids in both the ethanolic and aqueous leaf extracts, while phytosterols were only detected in the ethanolic extract. Folin-Ciocalteu assay determined the total phenolic content in gallic acid equivalents (GAE) to be 85.86 ± 3.71 and 32.32 ± 1.01 mg GAE/100 g dried sample for the ethanolic and aqueous extracts, respectively. The total phenolic content in quercetin equivalents (QE) was 74.44 ± 3.11 and 29.43 ± 0.85 mg QE/100g dried sample for the ethanolic and aqueous extracts, respectively. The radical scavenging activity of the different solvent fractions containing varying concentrations of the extract was determined using the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay. The ethyl acetate and 1-butanol fractions were found to have the highest radical scavenging activity. Mineral analysis via Energy Dispersive X-Ray Spectrometry (EDS) of the ash of G. levis leaves showed that Si is the major component, followed by K and Mg. These results point to the potential of G. levis leaves as a source of minerals and bioactive compounds with medicinal value.
Go to : 
REFERENCES
(1). Chongtham N., Bisht M. S., Haorongbam S.Compr. Rev. Food Sci. F. 2011; 10:153–168.
(2). Wang J., Yue Y. D., Tang F., Sun J.Molecules. 2012; 17:12297–12311.
(3). Keski-Saari S., Ossipov V., Julkunen-Tiitto R., Jia J., Danell K., Veteli T., Guiquan Z., Yaowu X., Niemel P.Biochem. Syst. Ecol. 2008; 36:758–765.
(4). Scurlock J. M. O., Dayton D. C., Hames B.Bamboo; an overlooked biomass resource; Oak Ridge National Laboratory: Oak Ridge, Tennessee. 2000; 34.
(5). Cunniff P., Horwitz W.Official Methods of Analysis of AOAC International. 16th ed.AOAC International;Gaithersburg: 1995.
(6). Harborne J. B.Phytochemical methods: A guide to modern techniques of plant analysis (3rd ed.); Chapman and Hall: New York, USA. 1998; 279.
(7). Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H.Internationale Pharmaceutica Sciencia. 2011; 1:98–106.
(8). Tongco J. V. V., Aguda R. M., Razal R. A. J.Chem. Pharm. Res. 2014; 6:709–713.
(9). Ragazzi E., Veronese G. J.Chromatogr. 1973; 77:369–375.
(10). Burda S., Oleszek W. J.Agric. Food Chem. 2001; 49:2774–2779.
(12). Oke O. L.Food Chem. 1980; 6:97–109.
(13). Supriyatin R. S., Sukmawati D.Asian J. Microbiol. Biotech. Env. Sci. 2015; 17:443–450.
Go to : 
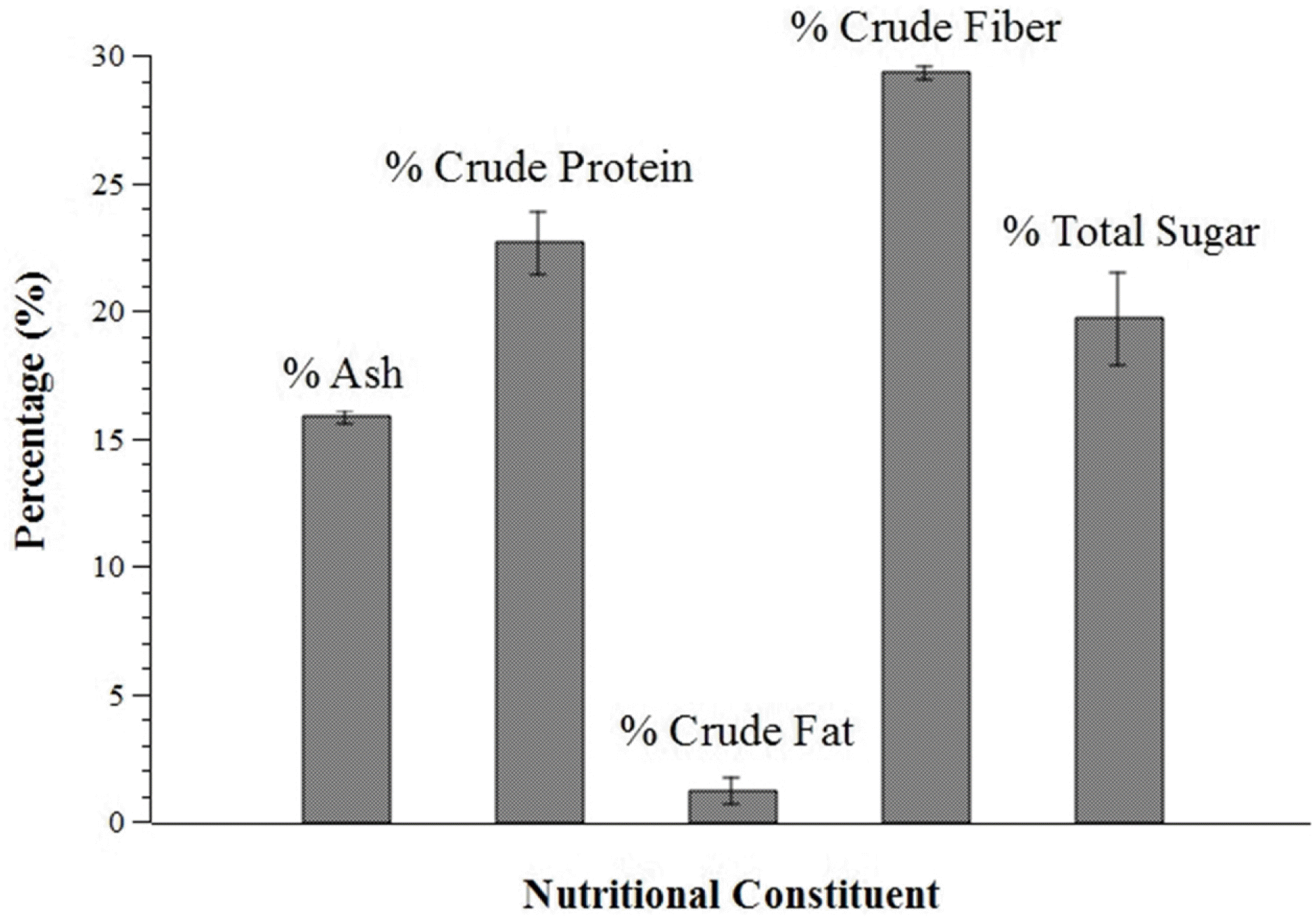 | Fig. 1.Nutritional constituents of Gigantochloa levis (Blanco) Merr. leaves. Error bars represent measured standard deviation. |
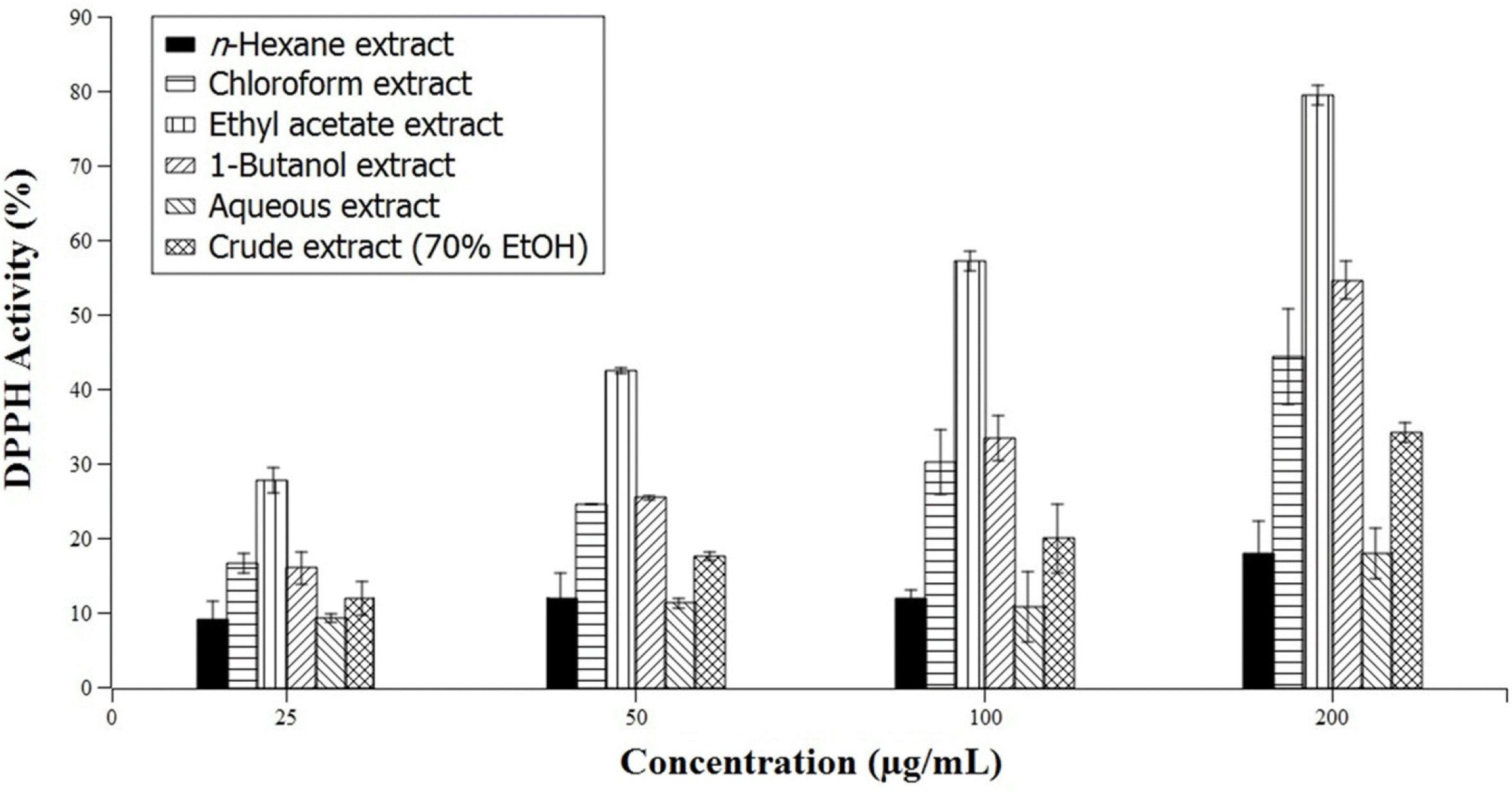 | Fig. 2.DPPH scavenging activity of the solvent fractions and crude extract of Gigantochloa levis (Blanco) Merr. leaves compared to gallic acid as standard (100%) in varying concentrations. Error bars represent measured standard deviation. |
Table 1.
Qualitative phytochemical screening of ethanolic and aqueous extracts of Gigantochloa levis (Blanco) Merr. leaves
Table 2.
Total phenolic contents (TPC) of the extracts of Gigantochloa levis (Blanco) Merr. leaves in terms of gallic acid and quercetin equivalents
| Ethanolic extract | Aqueous extract | |
|---|---|---|
| Gallic acid equivalents (mg GAE/100 g air-dried sample) | 85.86 ± 3.71 | 32.32 ± 1.01 |
| Quercetin equivalents (mg QE/100 g air-dried sample) | 74.44 ± 3.11 | 29.43 ± 0.85 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download