Abstract
Erectile dysfunction (ED) is a highly prevalent disorder that affects millions of men and considered to be an early symptom of atherosclerosis and a precursor of various systemic vascular disorders. The aim of the present study was to prepare ginsenoside Re enriched fraction (GS-F3K1, ginsenoside Re 10%, w/w) from ginseng berries flesh and to investigate the enhanced activities of GS-F3K1 on alcohol-induced ED. GS-F3K1 was prepared by the continuous liquid and solid separating centrifugation and circulatory ultrafiltration from ginseng berries flesh. GS-F3K1 was administered for 5 weeks in ethanol-induced ED rat by oral administration of 20% ethanol. To investigate the effects of GS-F3K1 on ED model, the levels of nitrite expression, cyclic guanosine monophosphate (cGMP) and erectile response of the penile corpus cavernosum of rat were measured. The erectile response of the corpus cavernosum was restored after GS-F3K1 administration, to a level similar to the normal group. The level of nitrite and cGMP expression in the corpus cavernosum of GS-F3K1-administered male rats was increased significantly compared to positive control group. GS-F3K1 from ginseng berries should effectively restore ethanol-induced ED in male rats and could be developed as a new functional food for the elderly men.
Go to : 
REFERENCES
(1). Huang K. C.The Pharmacology of Chinese Herbs: Boca Raton; CRC Press; USA. 1999; 17–45.
(2). Attele A. S., Wu J. A., Yuan C. S.Biochem. Pharmacol. 1999; 58:1685–1693.
(3). Jang H. J., Han I. H., Kim Y. J., Yamabe N., Lee D., Hwang G. S., Oh M., Choi K. C., Kim S. N., Ham J., Eom D. W., Kang K. S. J.Agric. Food Chem. 2014; 62:2830–2836.
(5). Cho K. S., Park C. W., Kim C. K., Jeon H. Y., Kim W. G., Lee S. J., Kim Y. M., Lee J. Y., Choi Y. D.Asian J. Androl. 2013; 15:503–507.
(6). Zhang H., Zhou Q. M., Li X. D., Xie Y., Duan X., Min F. L., Liu B., Yuan Z. G.Arch. Pharm. Res. 2006; 29:145–151.
(7). Zhang H., Zhou Q., Li X., Zhao W., Wang Y., Liu H., Li N.Mol. Reprod. Dev. 2007; 74:497–501.
(8). Nakaya Y., Mawatari K., Takahashi A., Harada N., Hata A., Yasui S. J.Med. Invest. 2007; 54:381–384.
(9). Seol S. Y., Kim B. R., Hong S. C., Yoo J. H., Lee K. H., Lee H. J., Park J. D., Pyo M. K.Nat. Prod. Sci. 2014; 20:58–64.
(10). Feldman H. A., Goldstein I., Hatzichristou D. G., Krane R. J., McKinlay J. B. J.Urol. 1994; 151:54–61.
(11). Sommer F., Goldstein I., Korda J. B. J.Sex. Med. 2010; 7:2346–2358.
(12). Bancroft J. H., Jones H. G., Pullan B. R.Behav. Res. Ther. 1996; 4:239–241.
(13). Sandroni P.Clin. Auton. Res. 2001; 11:303–307.
(14). Choi Y. D., Xin Z. C., Choi H. K.Int. J. Impot. Res. 1998; 10:37–43.
(15). Choi H. K, Seong D. H., Rha K. H.Int. J. Impot. Res. 1995; 7:181–186.
(16). Choi H. K., Choi Y. D.Proc.'99 Korea-Japan Ginseng Symp. 1999; 97–106.
(17). Kim S.W., Paick J. S. Kor. J.Androl. 1999; 17:23–28.
(18). Choi Y. D., Park C. W., Jang J., Kim S. H., Jeon H. Y., Kim W. G., Lee S. J., Chung W. S.Int. J. Impot. Res. 2012; 25:45–50.
(19). Boolell M., Allen M. J., Ballard S. A., Gepi-Attee S., Muirhead G. J., Naylor A. M., Osterloh I. H.Int. J. Impot. Res. 1996; 8:47–52.
(20). Lue T. F., Tanagho E. A. J.Urol. 1987; 137:829–836.
(21). Sullivan M. E., Thompson C. S., Dashwood M. R., Khan M. A., Jeremy J. Y., Morgan R. J., Mikhailidis D. P.Cardiovasc. Res. 1999; 43:658–665.
(22). Moncada S., Higgs A. N. Engl. J.Med. 1993; 329:2002–2012.
(23). Park K. H., Kim J. S., Noh E. M., Yu H. N., Kim S. Y., Kim S. Z., Kim S. M., Chung E. Y.Mol. Cell. Toxicol. 2013; 9:235–241.
(24). Rees D. D., Palmer R. M., Moncada S.Proc. Natl. Acad. Sci. USA. 1989; 86:3375–3378.
(25). Ponizovsky A. M. J.Sex. Med. 2008; 5:2347–2358.
Go to : 
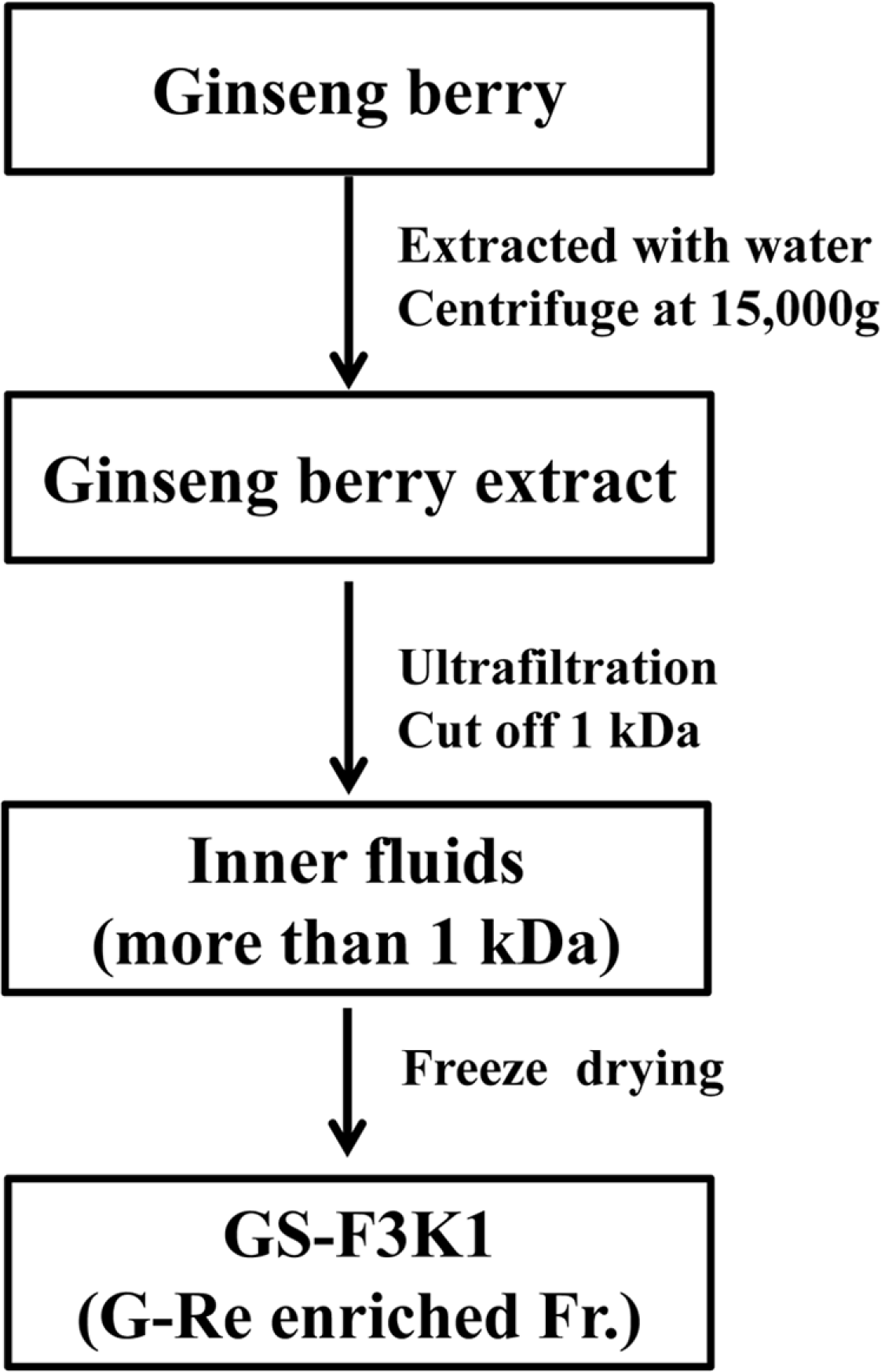 | Fig. 1.Schematic representation of the purification procedure of GS-F3K1 from ginseng berry. Gnsenoside Re enriched fraction (GS-F3K1) from ginseng berries flesh was prepared by a combination of continuous centrifugation and ultrafiltration to remove low molecular weight compounds. |
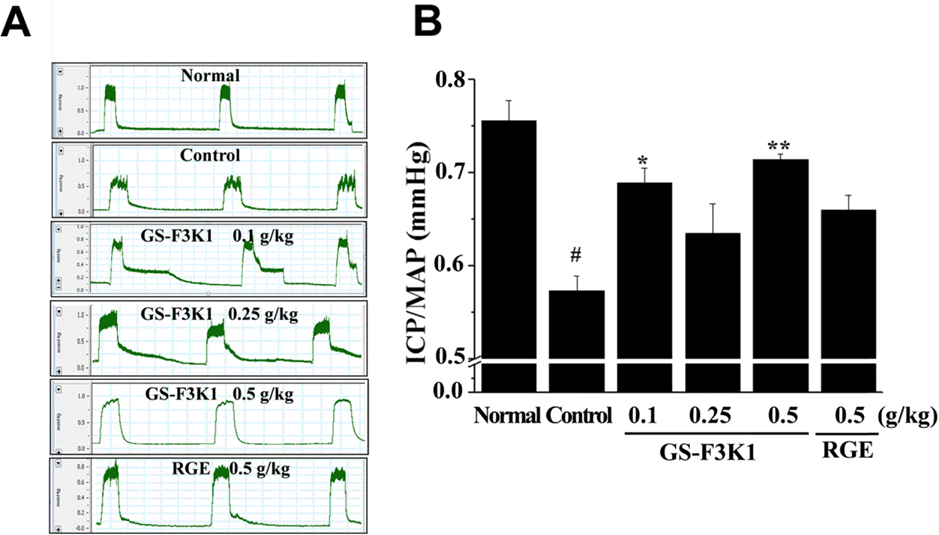 | Fig. 2.Effects of GS-F3K1 and RGE on the erectile response to carvernous nerve stimulation in ethanol-induced ED rats. (A) Oscillations of the intracavernous pressure (ICP), and (B) Statistic analysis. Data are given as mean of value ± SD (n=6) from three independent experiments. # p < 0.05 when compared with the normal group. ∗p < 0.05, ∗∗∗p < 0.001when compared with the control group by independent t test. |
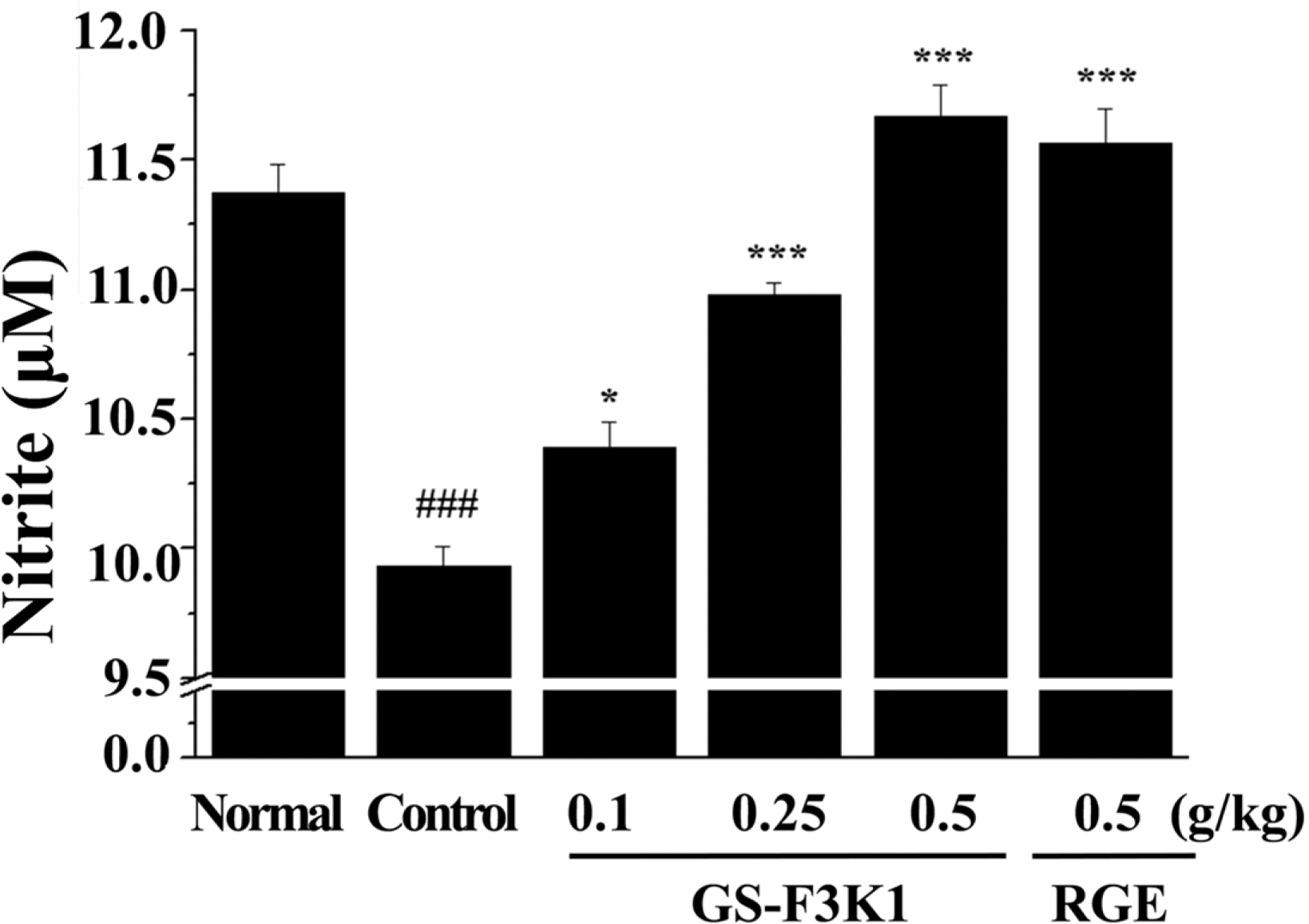 | Fig. 3.Effects of GS-F3K1 and RGE on nitrite production to carvernous nerve stimulation in ethanol-induced ED rats. Data are given as mean of value ± SD (n=6) from three independent experiments. ###p < 0.001 when compared with the normal group. ∗p <0.05, ∗∗∗p <0.001 when compared with control group by independent t test. |
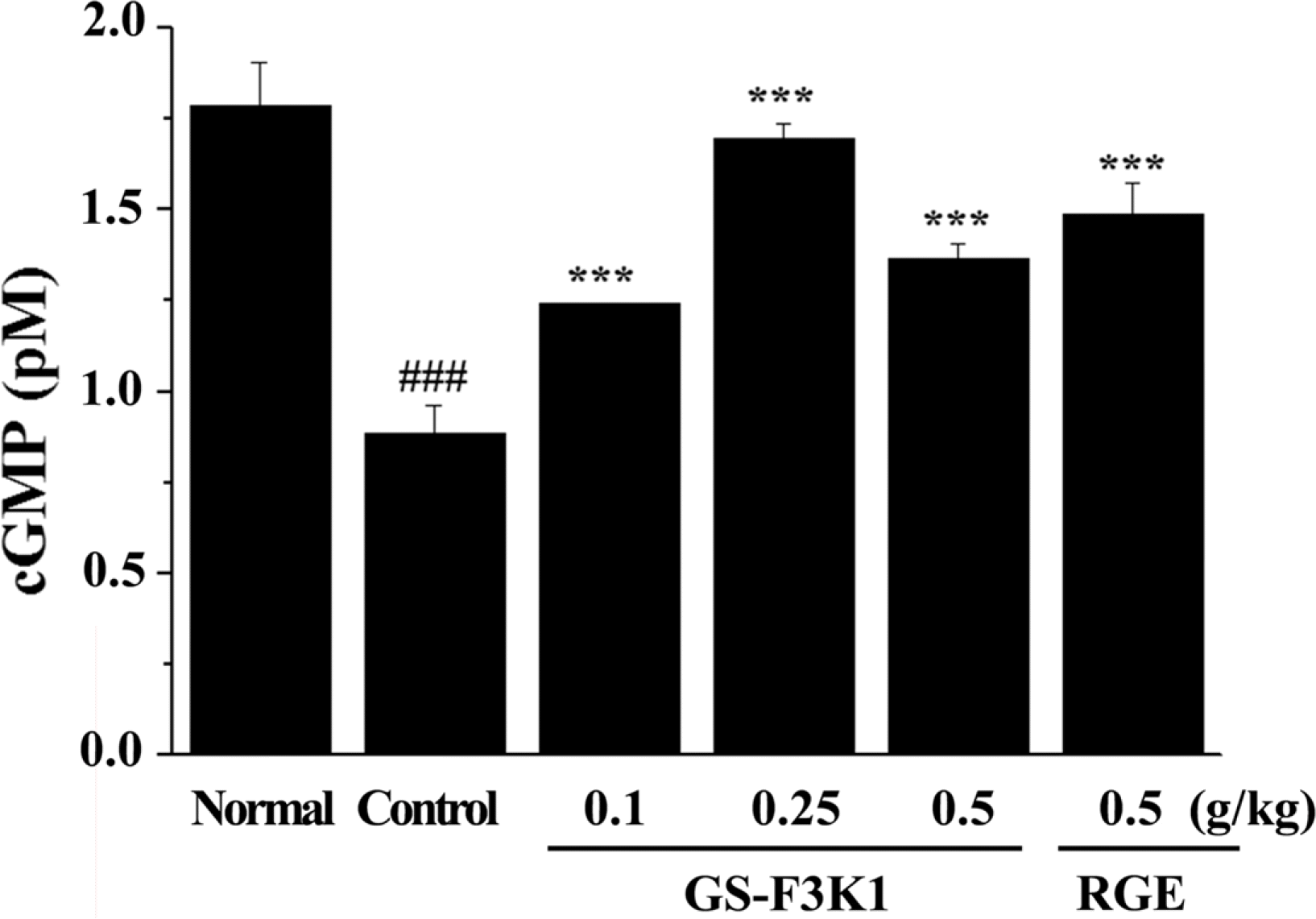 | Fig. 4.Effects of the GS-F3K1 and RGE on cGMP level to carvernous nerve stimulation in ethanol-induced ED rats. Data are given as mean of value ± SD (n=6) from three independent experiments. Level of significance was identified statistically using independent t test. ###p < 0.001 when compared with the normal group. ∗∗∗p <0.001 when compared with the control group by independent t test. |
Table 1.
Change in ginsenoside composition of ginseng berry extract by heat
| Ginsenoside (mg/g) | Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rd | S-Rg3 | R-Rg3 |
|---|---|---|---|---|---|---|---|---|---|
| FGBEa) | 4.7±0.23 | 50.1±2.51 | 1.4±0.07 | 8.1±0.41 | 3.3±0.17 | 3.9±0.20 | 4.0±0.20 | 0.1±0.00 | 0.0±0.00 |
| EGBEb) | 4.1±0.20 | 41.5±2.08 | 1.0±0.05 | 6.5±0.32 | 1.8±0.09 | 2.3±0.11 | 2.1±0.10 | 0.2±0.00 | 0.2±0.00 |
| HGBEc) | 1.7±0.09 | 20.0±1.00 | 1.1±0.05 | 6.6±0.33 | 1.1±0.12 | 1.4±0.32 | 1.5±0.37 | 2.5±0.12 | 3.0±0.15 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download