Abstract
Extraction and fractionation of Pulsatilla koreana flowers followed by, repeated open column chromatography for EtOAc and n-BuOH fractions yielded four flavonoid glycosides, namely, astragalin (1), tiliroside (2), buddlenoide A (3), and apigenin-7-O-(3”-E-p-coumaroyl)-glucopyranoside (4). The chemical structures of these flavonoid glycosides were elucidated on the basis of various spectroscopic methods including electronic ionization mass spectrometry (EI-MS), 1D NMR (1H,13C, DEPT), 2D NMR (gCOSY, gHSQC, gHMBC), and infrared (IR) spectrometry. This study represents the first report of the isolation of the flavonoid glycosides from the flowers of P. koreana.
Go to : 
REFERENCES
(1). Jung B. S., Shin M. K.Hyang Yak Dae Sa Jeon; Young Lim Sa Publisher: Seoul. 2003; 495.
(2). Kim Y., Kim S. B., You Y. J., Ahn B. Z.Planta Med. 2002; 68:271–274.
(3). Li W., Yan X. T., Sun Y. N., Ngan T. T., Shim S. H., Kim Y. H.Biomol. Ther. 2014; 22:334–340.
(4). Bang S. C., Lee J. H., Song G. Y., Kim D. H., Yoon M. Y., Ahn B. Z.Chem. Pharm. Bull. 2005; 53:1451–1454.
(5). Li W., Ding Y., Sun Y. N., Yan X. T., Yang S. Y., Choi C. W., Kim E. J., Kang H. K., Kim Y. H.Arch. Pharm. Res. 2013; 36:768–774.
(6). Li W., Ding Y., Sun Y. N., Yan X. T., Yang S. Y., Choi C. W., Cha J. Y., Lee Y. M., Kim Y. H.Chem. Pharm. Bull. 2013; 61:471–476.
(7). Cho S. C., Sultan M. Z., Moon S. S.Arch. Pharm. Res. 2009; 32:489–494.
(8). Cuong T. D., Hung T. M., Lee M. K., Thao N. T. P., Jang H. S., Min B. S.Nat. Prod. Sci. 2009; 15:250–255.
(9). Liu Q., Ahn J. H., Kim S. B., Hwang B. Y., Lee M. K.Planta Med. 2012; 78:1783–1786.
(10). Gronquist M., Bezzerides A., Attygalle A., Meinwald J., Eisner M., Eisner T.Proc. Natl. Acad. Sci. USA. 2001; 98:13745–13750.
(11). Moon M. Y., Baik J. S., Kim S. S., Jang W. J., Kim M. S., Lee N. H. J.Soc. Cosmet. Sci. Korea. 2009; 35:251–256.
(12). Oshima Y., Nakabayashi T.Nippon Nogei Kagaku Kaishi J. 1953; 27:756–759.
(13). Hörhammer L., Stich L., Wagner H.Naturwissenschaften J. 1959; 46:358.
(14). Kubo I., Yokokawa Y.Phytochemistry. 1992; 31:1075–1077.
(15). Subramani K., Karnan R., Sajitha M., Anbarasan P. M.Indian J. 2013; 7:102–106.
(16). Yoo N. H., Jang D. S., Yoo J. L., Lee Y. M., Kim Y. S., Cho J. H., Kim J. S. J.Nat. Prod. 2008; 71:713–715.
(17). Jeong H. J., Ryu Y. B., Park S. J., Kim J. H., Kwon H. J., Kim J. H., Park K. H., Rho M. C., Lee W. S.Bioorg. Med. Chem. 2009; 17:6816–6823.
(18). Luyen B. T. T., Tai B. H., Thao N. P., Kim J. E., Cha J. Y., Xin M. J., Lee Y. M., Kim Y. H.Bioorg. Med. Chem. Lett. 2014; 24:1895–1900.
(19). Tsukamoto S., Tomise K., Aburatani M., Onuki H., Hirorta H., Ishiharajima E., Ohta T. J.Nat. Prod. 2004; 67:1839–1841.
(20). Itoh T., Ninomiya M., Yasuda M., Koshikawa K., Deyashiki Y., Nozawa Y., Akao Y., Koketsu M.Bioorg. Med. Chem. 2009; 17:5374–5379.
(21). Qin N., Li C. B., Jin M. N., Shi L. H., Duan H. Q., Niu W. Y.Eur. J. Med. Chem. 2011; 46:5189–5195.
(22). Kubo I., Yokokawa Y., Kinst-Hori I. J.Nat. Prod. 1995; 58:739–743.
(23). Argyropoulou A., Samara P., Tsitsilonis O., Skaltsa H.Phytother. Res. 2012; 26:1800–1806.
Go to : 
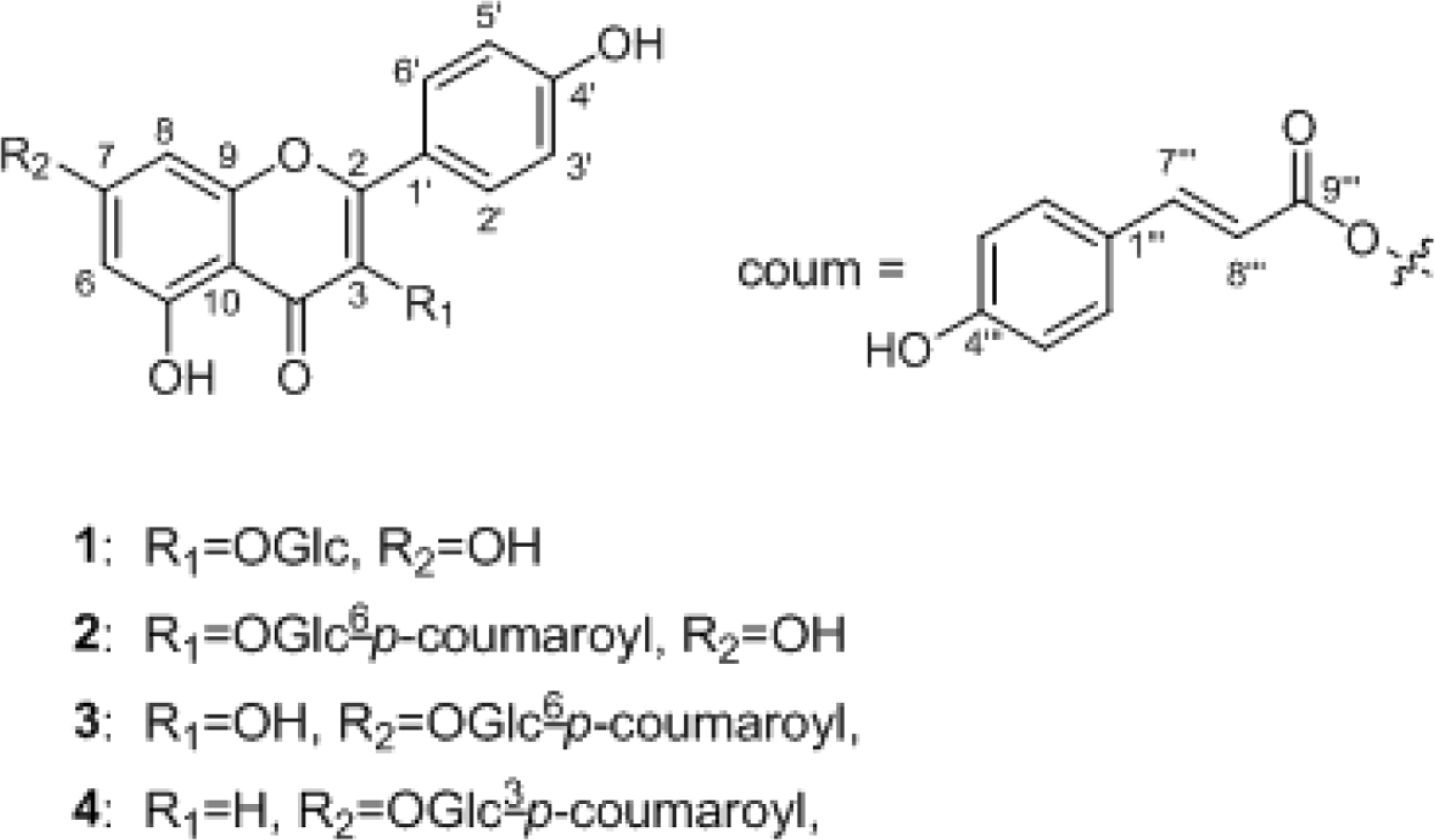 | Fig. 1.Chemical structures of compounds 1 − 4 from the flowers of Pulsatilla koreana. OGlc: O-β-D-glucopyranosyl; Glc: β-D-glucopyranosyl; coum: p-coumaroyl. |
Table 1.
1 H- (400MHz, coupling pattern, J in Hz) and13 C-NMR (100MHz) data of compounds 1–4 from the flowers of Pulsatilla koreana
| 1a | 2b | 3a | 4a | |||||
|---|---|---|---|---|---|---|---|---|
| No. | δH | δC | δH | δC | δH | δC | δH | δC |
| 2 | 158.0 | 157.6 | 164.5 | 164.9 | ||||
| 3 | 135.3 | 134.9 | 133.7 | 6.47 (s) | 105.0 | |||
| 4 | 179.2 | 178.6 | 177.9 | 182.6 | ||||
| 5 | 162.7 | 162.2 | 156.9 | 163.3 | ||||
| 6 | 6.19 (d, 2.0) | 99.8 | 6.68 (br.s) | 99.9 | 6.12 (d, 2.0) | 98.5 | 6.62 (br.s) | 99.6 |
| 7 | 165.7 | 166.3 | 164.9 | 165.3 | ||||
| 8 | 6.37 (d, 2.0) | 94.8 | 6.68 (br.s) | 94.8 | 6.29 (d, 2.0) | 93.3 | 6.62 (br.s) | 94.5 |
| 9 | 158.8 | 157.7 | 157.9 | 157.5 | ||||
| 10 | 105.6 | 105.8 | 104.1 | 105.6 | ||||
| 1′ | 122.5 | 121.8 | 121.2 | 121.4 | ||||
| 2′ | 8.05 (d, 9.2) | 132.1 | 8.41 (d, 8.4) | 131.8 | 7.98 (d, 8.8) | 129.7 | 7.86 (d, 7.6) | 128.2 |
| 3′ | 6.89 (d, 9.2) | 115.9 | 7.20 (d, 8.4) | 116.0 | 6.79 (d, 8.8) | 115.3 | 6.92 (d, 7.6) | 115.6 |
| 4′ | 161.2 | 161.7 | 160.0 | 161.7 | ||||
| 5′ | 6.89 (d, 9.2) | 115.9 | 7.20 (d, 8.4) | 116.0 | 6.79 (d, 8.8) | 115.3 | 6.92 (d, 7.6) | 115.6 |
| 6′ | 8.05 (d, 9.2) | 132.1 | 8.41 (d, 8.4) | 131.8 | 7.98 (d, 8.0) | 129.7 | 7.86 (d, 7.6) | 128.2 |
| 1″ | 5.21 (d, 7.6) | 104.0 | 6.19 (d, 6.4) | 104.1 | 5.39 (d, 7.6) | 102.5 | 5.58 (d, 7.6) | 102.6 |
| 2″ | 3.43 (dd, 8.4, 7.6) | 75.6 | 4.17 (overlapped) | 75.9 | 3.44 (overlapped) | 74.2 | 3.52 (overlapped) | 73.2 |
| 3″ | 3.43 (overlapped) | 78.2 | 4.35 (overlapped) | 78.3 | 3.48 (overlapped) | 76.5 | 3.68 (overlapped) | 76.4 |
| 4″ | 3.43 (overlapped) | 71.2 | 4.17 (overlapped) | 71.2 | 3.42 (overlapped) | 70.2 | 3.49 (overlapped) | 69.8 |
| 5″ | 3.21 (m) | 78.0 | 4.35 (overlapped) | 75.9 | 3.46 (overlapped) | 74.3 | 3.29 (overlapped) | 76.9 |
| 6″′ | 3.69 (dd, 12.0, 2.4) | 62.4 | 4.97 (br.d, 11.6) | 64.4 | 4.31 (dd, 12.0, 2.4) | 63.8 | 3.94 (br.d, 12.0) | 62.8 |
| 6″′ | 3.53 (dd, 12.0, 5.6) | 4.83 (dd, 11.6, 5.6) | 4.20 (dd, 12.0, 5.8) | 3.70 (overlapped) | ||||
| 1″′ | 126.0 | 125.6 | 125.6 | |||||
| 2″′ | 7.48 (d, 8.4) | 130.6 | 7.30 (d, 8.8) | 130.7 | 7.48 (d, 8.4) | 129.6 | ||
| 3″′ | 7.14 (d, 8.4) | 116.7 | 6.81 (d, 8.8) | 114.5 | 6.82 (d, 8.4) | 115.3 | ||
| 4″′ | 161.3 | 159.7 | 159.8 | |||||
| 5″′ | 7.14 (d, 8.4) | 116.7 | 6.81 (d, 8.8) | 114.5 | 6.82 (d, 8.4) | 115.3 | ||
| 6″′ | 7.48 (d, 8.4) | 130.6 | 7.30 (d, 8.8) | 130.7 | 7.48 (d, 8.4) | 129.6 | ||
| 7″′ | 7.82 (d, 16.0) | 145.1 | 7.40 (d, 16.0) | 145.1 | 7.61 (d, 16.0) | 144.0 | ||
| 8″′ | 6.48 (d, 16.0) | 114.8 | 6.08 (d, 16.0) | 113.2 | 6.33 (d, 16.0) | 113.4 | ||
| 9″′ | 167.2 | 167.3 | 168.3 | |||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download