Abstract
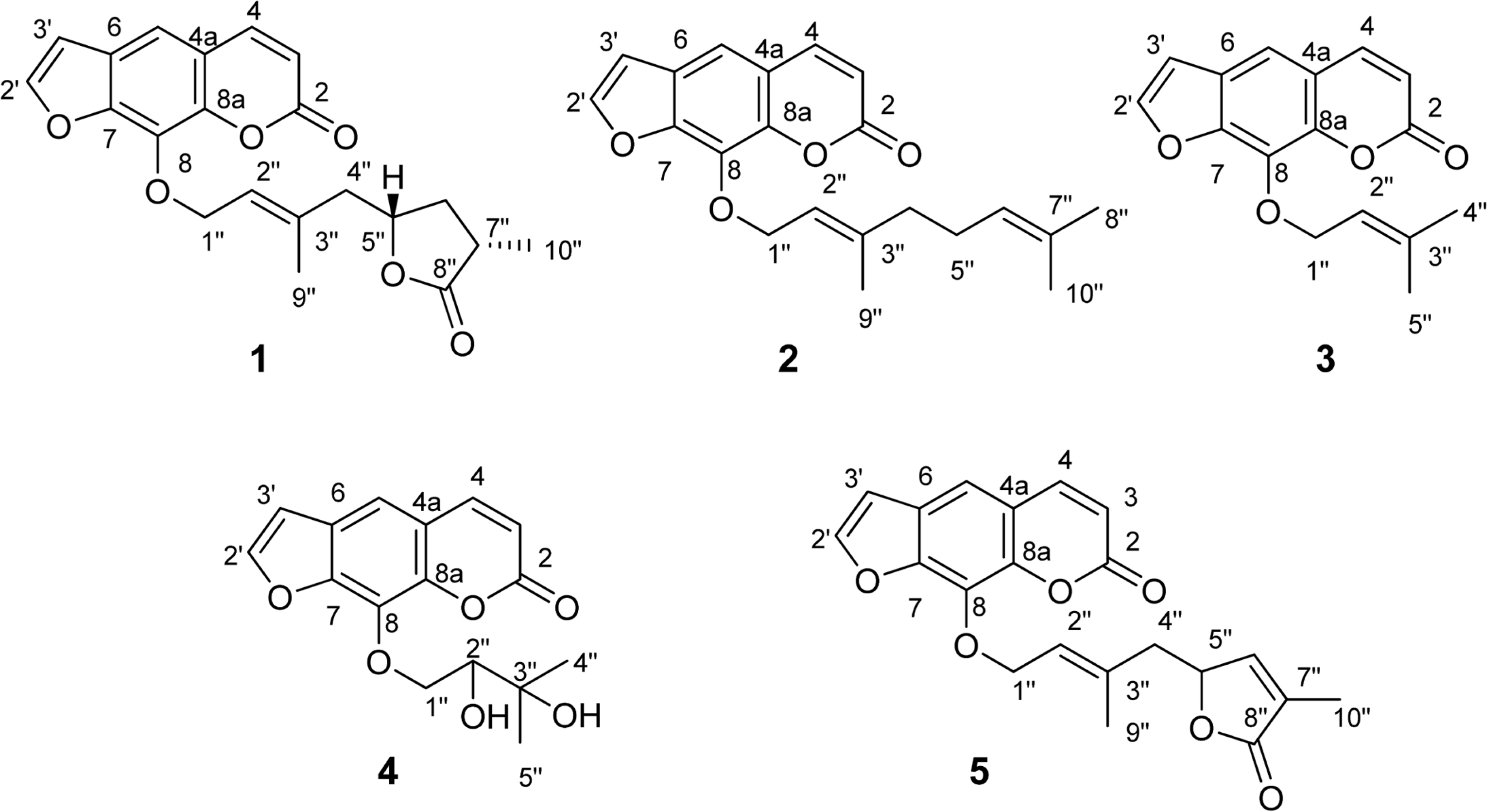
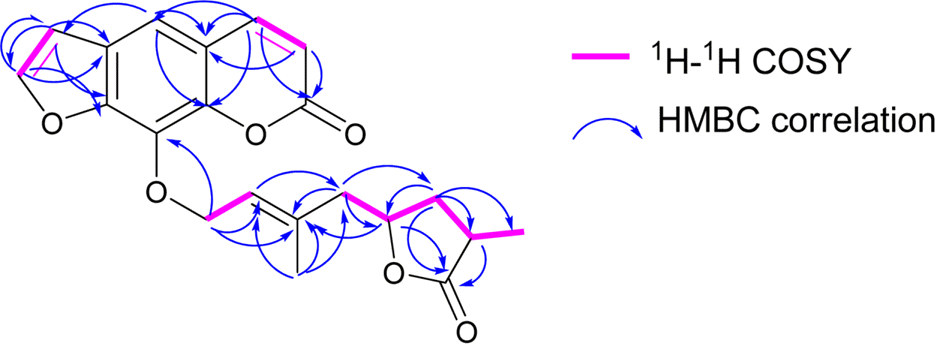
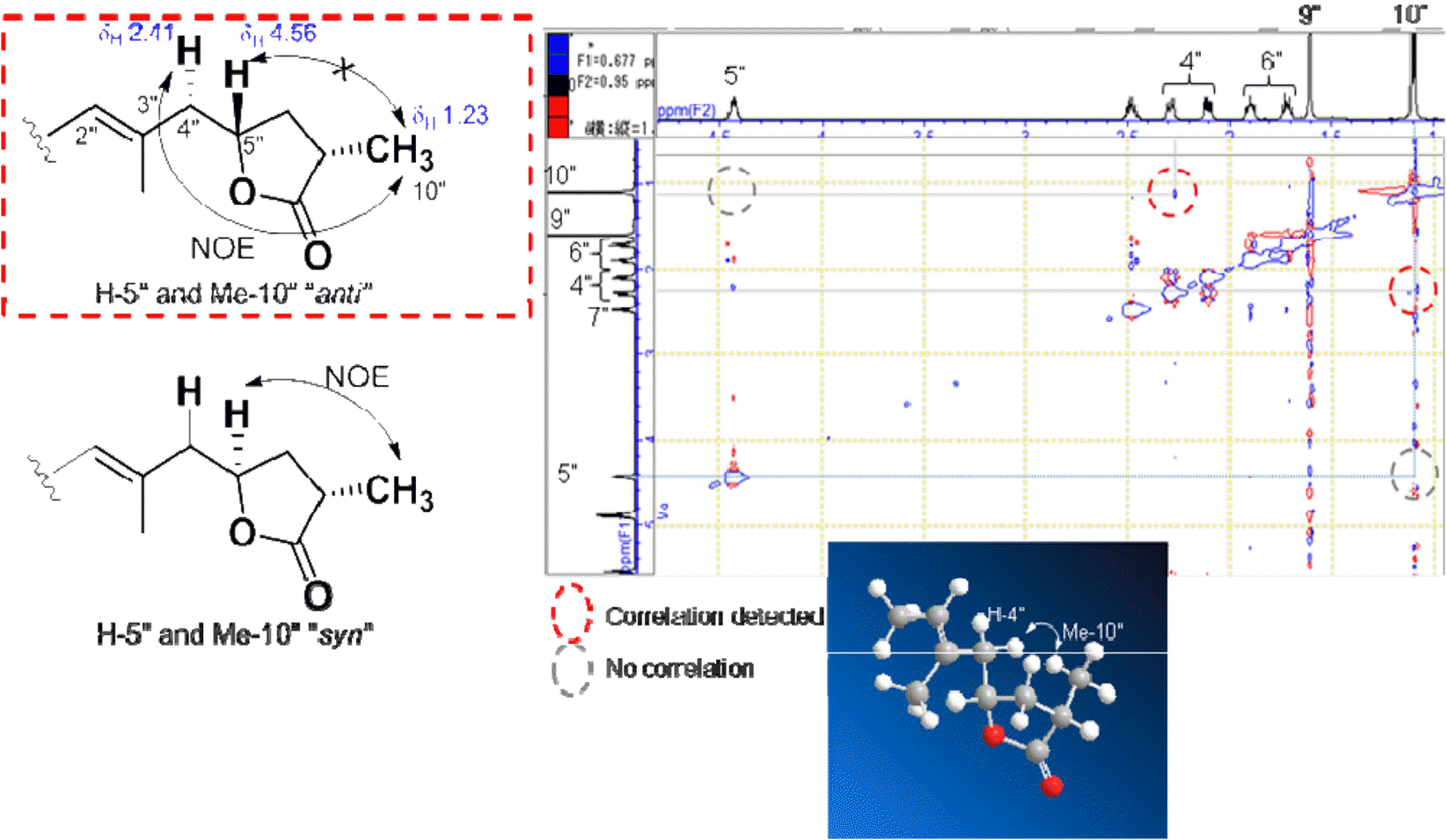
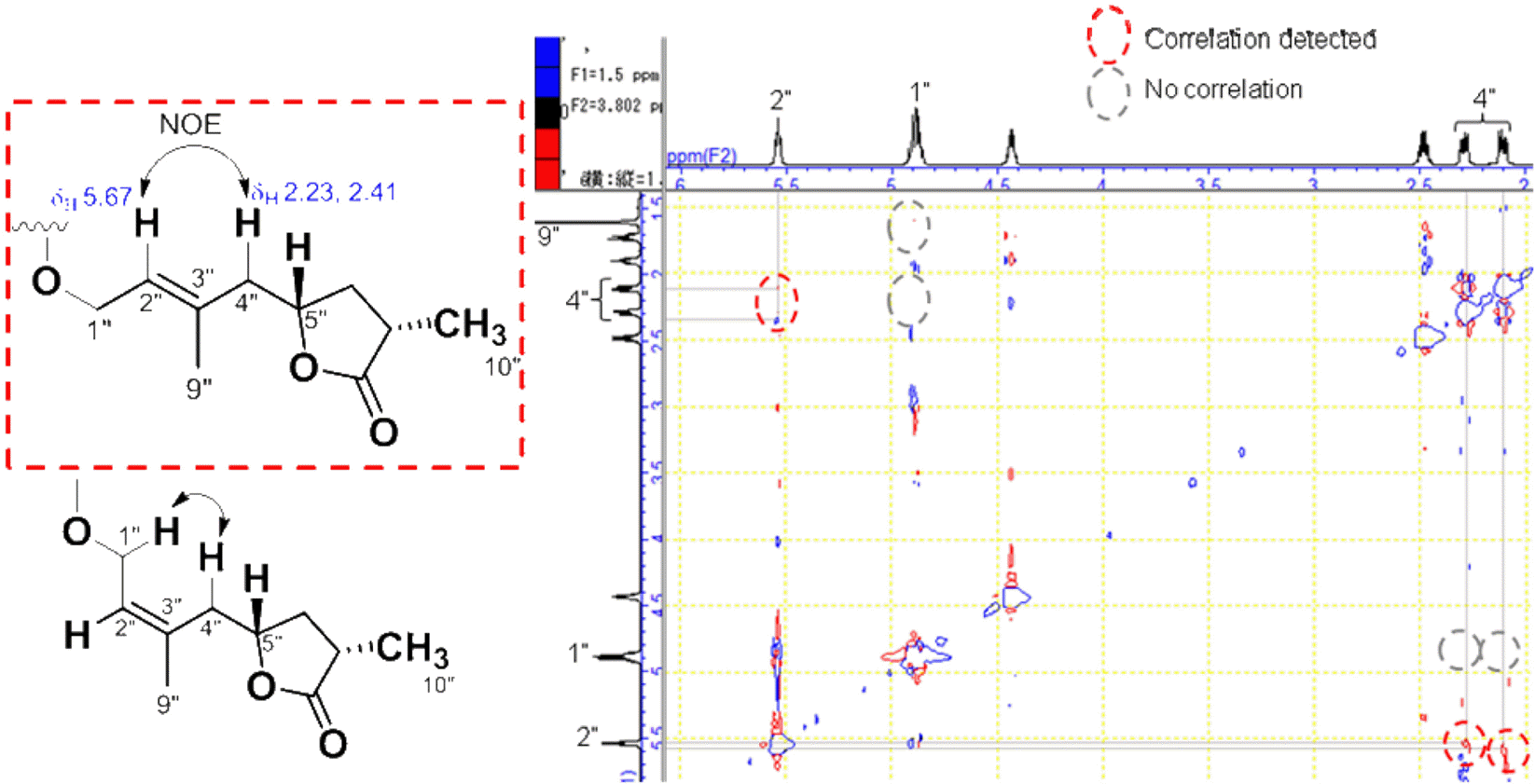
The first study on chemical constituents and biological activities of Clausena lansium (Lour.) Skeels (Rutaceae) growing in Vietnam has been done. Phytochemical investigation of n-hexane extract led to the isolation of five compounds: dihydroindicolactone (1), 8-geranyloxy psoralen (2), imperatorin (3), heraclenol (4) and indicolactone (5), in which this is the first report on the presence of dihydroindicolactone (1). Their structures were elucidated based on LC/MS/NMR hyphenated techniques as well as comparison with those of literature data. The n-hexane extract and its subfractions, ethanol 95% extract and several isolated compounds were evaluated for antifungal activity.
Go to : 
REFERENCES
(1). Chi V. V.The dictionary of Vietnamese medicinal Plants; Medicine Publishing House : Vietnam. 1999; 1148.
(2). Adebajo A. C., Iwalewa E. O., Obuotor E. M., Ibikunle G. F., Omisore N. O., Adewunmi C. O., Obaparusi O. O., Klaes M., Adetogun G. E., Schmidt T. J., Verspohl E. J. J.Ethnopharmacol. 2009; 122:10–19.
(3). Prasad K. N., Xie H., Hao J., Yang B., Qiu S., Wei X., Chen F., Jiang Y. J.Food Chem. 2010; 118:62–66.
(4). Chokeprasert P., Charles A. L., Sue K. H., Huang T. C. J.Food Comp. Anal. 2007; 20:52–56.
(5). Dincel D., Hatipoglu S. D., Goren A. C., Topcu G. Turk. J.Chem. 2013; 37:675–683.
(6). Lakshmi V., Prakash D., Raj K., Kapil R. S., Popli S. P.Phytochemistry. 1984; 23:2629–2631.
(7). Maneerat W., Prawat U., Saewan N., Laphookhieo S. J.Braz. Chem. Soc. 2010; 21:665–668.
(8). Takamasa K., Koichi M., Michiko A., Ryoko S., Yuka M., Rui K., Atsuhiko H., Takashi S., Shuichi S., Shinichi W., Hideyo Y., Shigeru A., Noboru O. J.Clin. Microbiol. 2007; 45:3737–3742.
(9). Shen D. Y., Chan Y. Y., Hwang T. L., Juang S. H., Huang S. C., Kuo P. C., Thang T. D., Lee E. J., Damu A. G., Wu T. S. J.Nat. Prod. 2014; 77:1215–1223.
(10). Adams M., Ettl, S.;Kunert, O.;Wube A. A., Haslinger E., Bucar, F.;Bauer R.Planta Med. 2006; 72:1132–1135.
(11). Singh S., Singh P., Singh S. K., Trivedi M., Dixit R. K., Shanker P. Int. Res. J.Pharm. App. Sci. 2013; 3:1–11.
(12). Adikaram N. K. B., Abhayawardhane Y., Leslie Gunatilaka A. A., Ratnayake Bandara B. M., Kithsiri Wijeratne E. M.Plant Pathology. 1989; 38:258–265.
Go to : 
Table 1.
1 H (600 MHz) and13 C (150 MHz) NMR spectral data of compound 1–5 in CDCl3




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download