Abstract
References
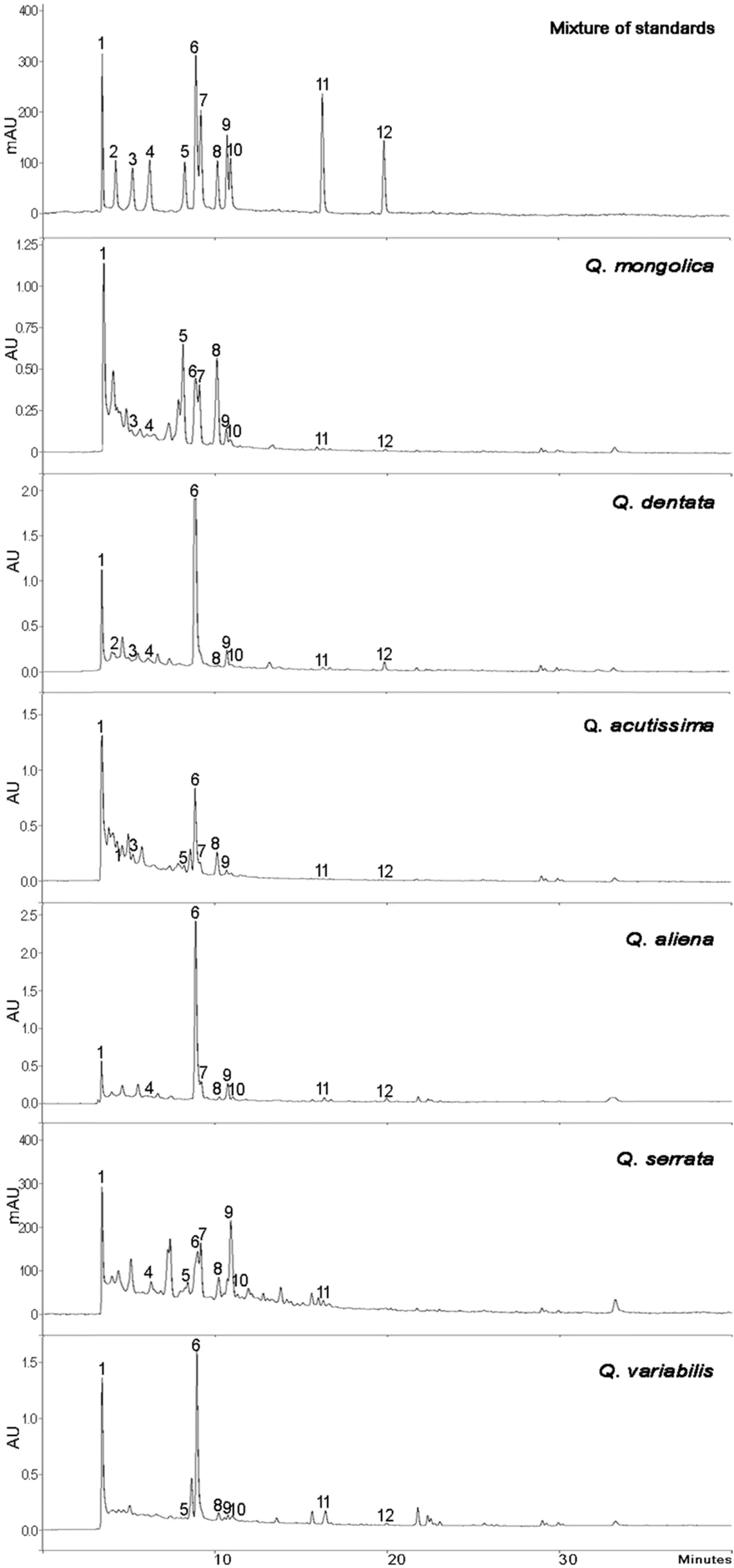
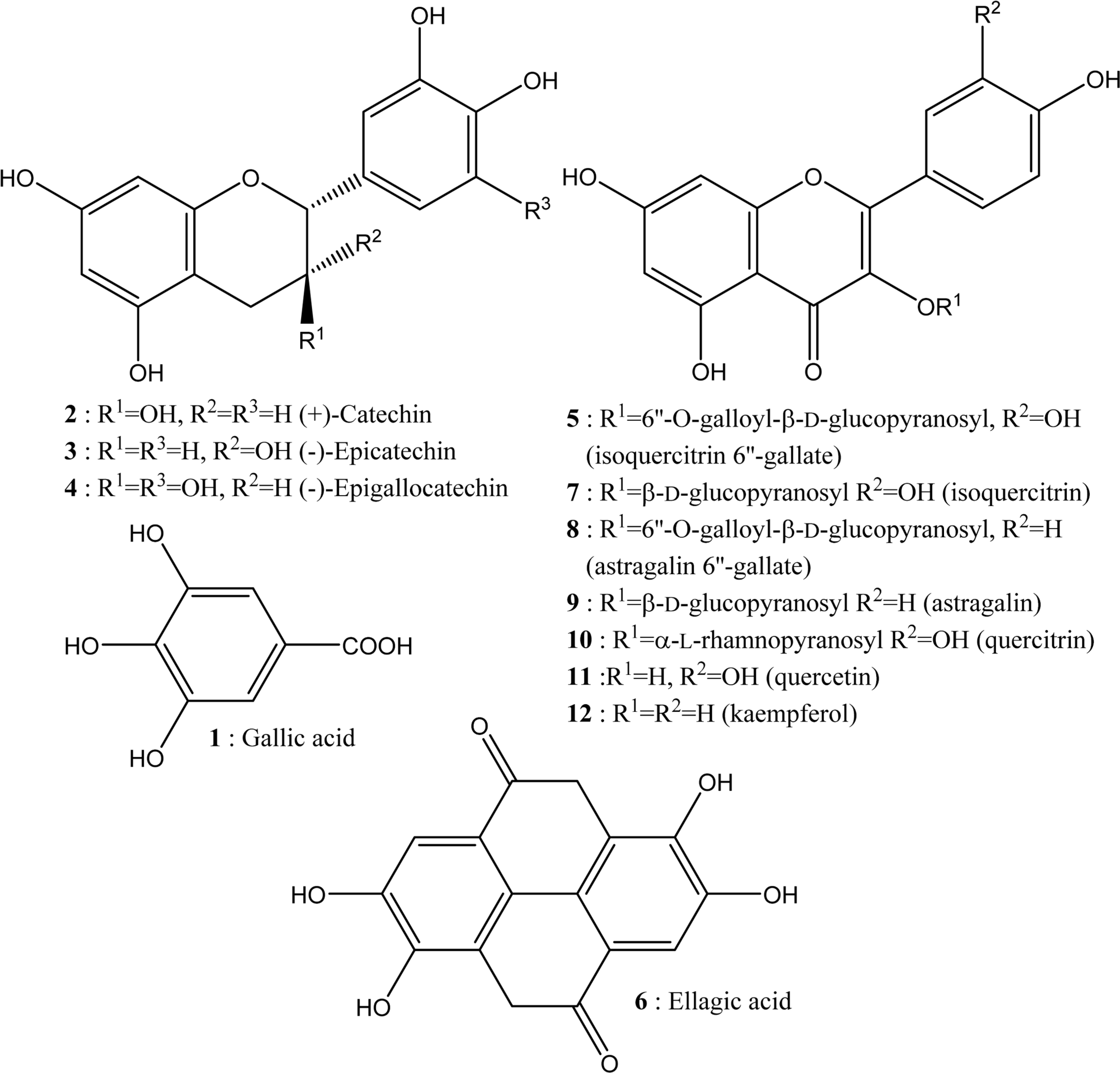 | Fig. 1.Structure of the twelve phenolic compounds used for the analysis of Quercus species. |
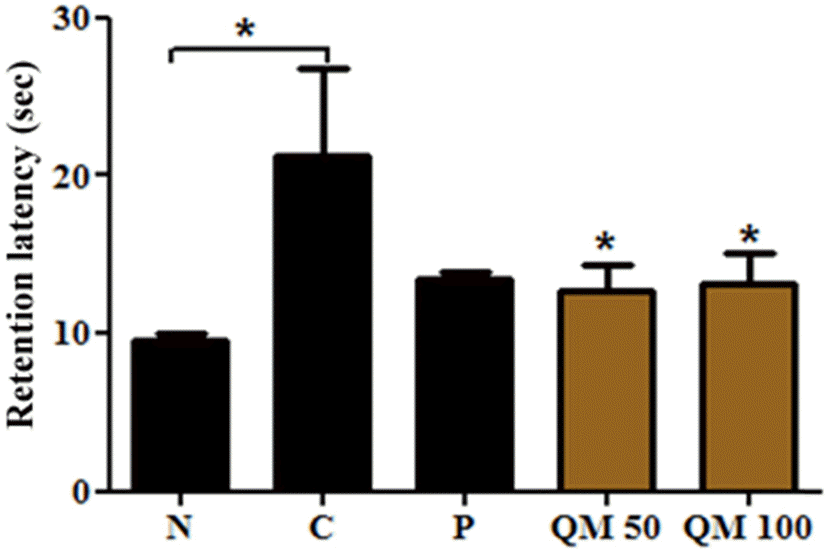 | Fig. 3.Effect of the extract of Q. mongolica leaves on the retention latency in passive avoidance test. QM 50 and QM 100 represent the group of mice treated with 50 and 100 mg/kg dose, respectively. The retention test was performed 24 h after the training trial. Normal group (N) of mice without any treatment (n = 5); Control group (C) was intraperitoneally injected with 1 mg/kg of scopolamine (n = 5); The positive control group (P) was injected with donepezil (x mg/kg); The two treatment groups, QM 50 and QM 100, were orally administered for 4 weeks before the training trial. Bars represent means ± SEM of retention latency. ∗ p < 0.05 vs. the C. |
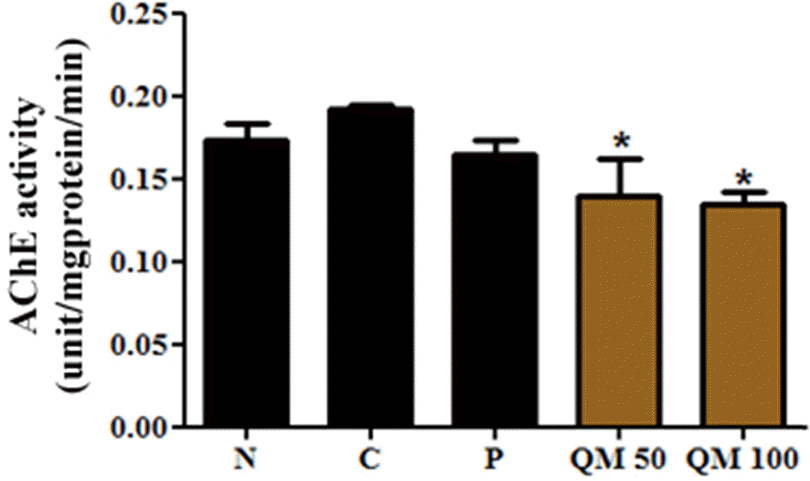 | Fig. 4.Acetylcholinesterase inhibitory activity of the extract of Q. mongolica leaves at the 50 (QM 50) and 100 mg/kg (QM 100) dose. Acetylcholinesterase inhibitory activity of the extract of Q. mongolica leaves at the 50 (QM 50) and 100 mg/kg (QM 100) dose. ∗p < 0.001 vs. C. |
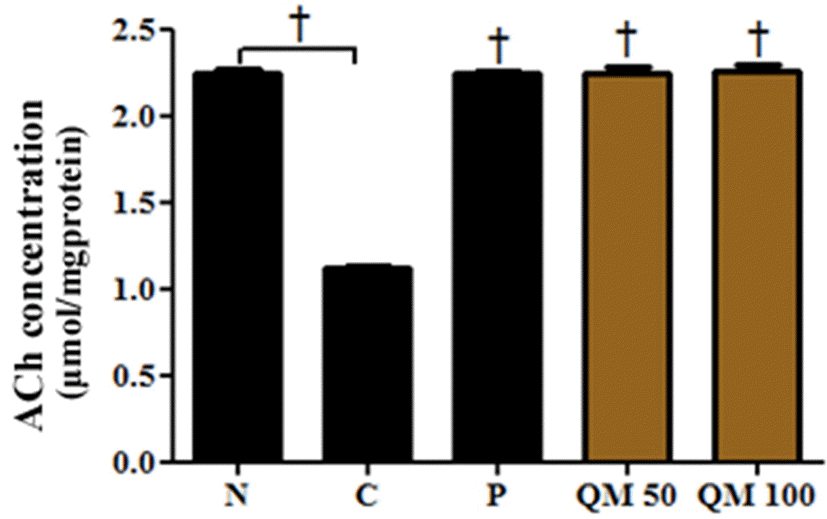 | Fig. 5.Effect of the extract of Q. mongolica leaves on the concentration of acetylcholine in scopolamine-treated mice. Effect of the extract of Q. mongolica leaves on the concentration of acetylcholine in scopolamine-treated mice. †p < 0.001 vs. C. |
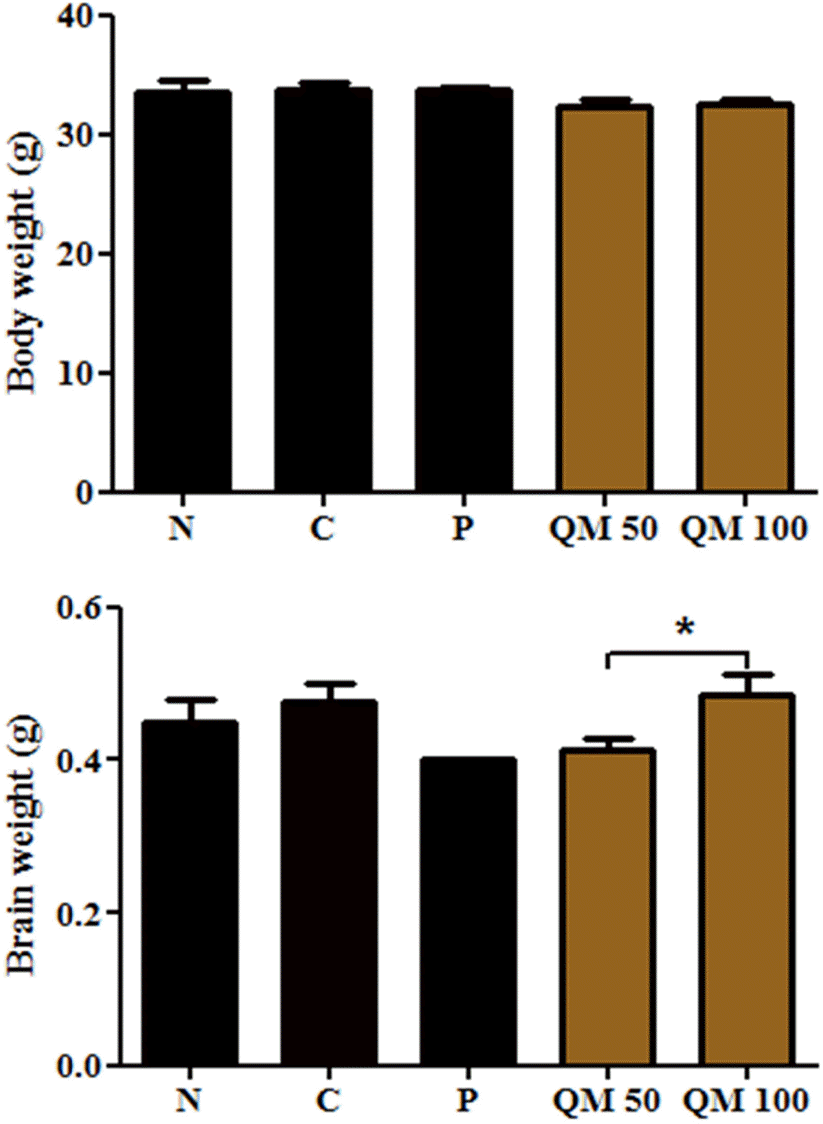 | Fig. 6.Effect of the extract of Q. mongolica leaves on body and brain weight in scopolamine-treated mice. |
Table 1.
| Compound | Equation of the linear regressiona | Linear range (µg/mL) | R2 b | tR | LODc (µg/mL) | LOQd (µg/mL) |
|---|---|---|---|---|---|---|
| Gallic acid (1) | y = 321.92x + 78.01 | 3.13–100.0 | 0.9998 | 3.41 | 0.13 | 0.44 |
| (−)-Epigallocatechin (2) | y = 44.059x + 24.48 | 15.6–500.0 | 0.9996 | 4.21 | 2.17 | 7.23 |
| (+)-Catechin (3) | y = 43.089x + 30.85 | 15.6–500.0 | 0.9995 | 5.21 | 2.07 | 6.90 |
| (−)-Epicatechin (4) | y = 60.405x + 43.58 | 15.6–500.0 | 0.9996 | 6.21 | 1.26 | 4.21 |
| Isoquercitrin6''-gallate (5) | y = 166.69x + 73.31 | 3.13–100.0 | 0.9998 | 8.25 | 0.28 | 0.93 |
| Ellagic acid (6) | y = 1651.8x + 94.63 | 0.78–25.00 | 0.9991 | 8.94 | 0.03 | 0.10 |
| Isoquercitrin (7) | y = 472.75x + 75.62 | 1.56–50.00 | 0.9997 | 9.19 | 0.09 | 0.31 |
| Astragalin 6''-gallate (8) | y = 255.79x + 59.05 | 3.13–100.0 | 0.9998 | 10.17 | 0.24 | 0.80 |
| Astragalin (9) | y = 459.93x + 56.94 | 1.56–50.00 | 0.9997 | 10.72 | 0.14 | 0.50 |
| Quercitrin (10) | y = 456.52x + 72.11 | 1.56–50.00 | 0.9998 | 10.93 | 0.11 | 0.35 |
| Quercetin (11) | y = 663.43x + 79.10 | 1.56–50.00 | 0.9996 | 16.31 | 0.06 | 0.21 |
| Kaempferol (12) | y = 460.36x + 63.79 | 1.56–50.00 | 0.9997 | 19.89 | 0.12 | 0.41 |
Table 2.
Recovery tests were performed on the extract of Q. mongolica spiked with each standard compound except for (−)-epigallocatechin. The tests of (−)-epigallocatechin were performed on the extract of Q. dentata. Relative standard deviation (RSD) values of precision tests were calculated for both retention time (tR) and peak area of threeindependent experiments.
Table 3.
The sign (−) indicates that the compound cannot be quantified (< LOQ) or not detected (< LOD) under 254 nm UV wavelength. Compounds: 1 (gallic acid), 2 ((−)-epigalocatechin), 3 ((+)-catechin), 4 ((−)-epicatechin), 5 (isoquercitrin 6''-gallate), 6 (ellagic acid), 7 (isoquercitrin), 8 (astragalin 6''-gallate), 9 (astragalin), 10 (quercitrin), 11 (quercetin), and 12 (kaempferol).
Table 4.
| Extract | Concentration (µg/ml) | IC50 µg/ml | |||
|---|---|---|---|---|---|
| 0.08 | 0.4 | 2 | 10 | ||
| Q. acutissima | 12.41 ± 0.46a | 31.07 ± 0.45 | 67.37 ± 0.18 | 91.93 ± 0.27 | 1.316 ± 0.011 |
| Q. alienta | 16.76 ± 2.67 | 32.84 ± 3.54 | 59.19 ± 2.00 | 88.37 ± 0.12 | 1.503 ± 0.117 |
| Q. dentata | 12.41 ± 0.98 | 20.59 ± 2.06 | 56.07 ± 0.62 | 89.01 ± 0.07 | 1.727 ± 0.037 |
| Q. mongolica | 31.06 ± 0.01 | 48.02 ± 0.62 | 70.68 ± 0.21 | 87.55 ± 0.36 | 0.831 ± 0.015 |
| Q. serrata | 57.81 ± 1.04 | 24.23 ± 1.21 | 57.10 ± 0.65 | 87.63 ± 0.25 | 1.672 ± 0.012 |
| Q. variabilis | 15.49 ± 1.19 | 24.29 ± 0.36 | 63.59 ± 0.07 | 89.35 ± 0.15 | 1.451 ± 0.001 |
| L-penicillamine | − | 38.92 ± 0.07 | 73.74 ± 0.86 | 91.37 ± 0.07 | 0.910 ± 0.015 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download