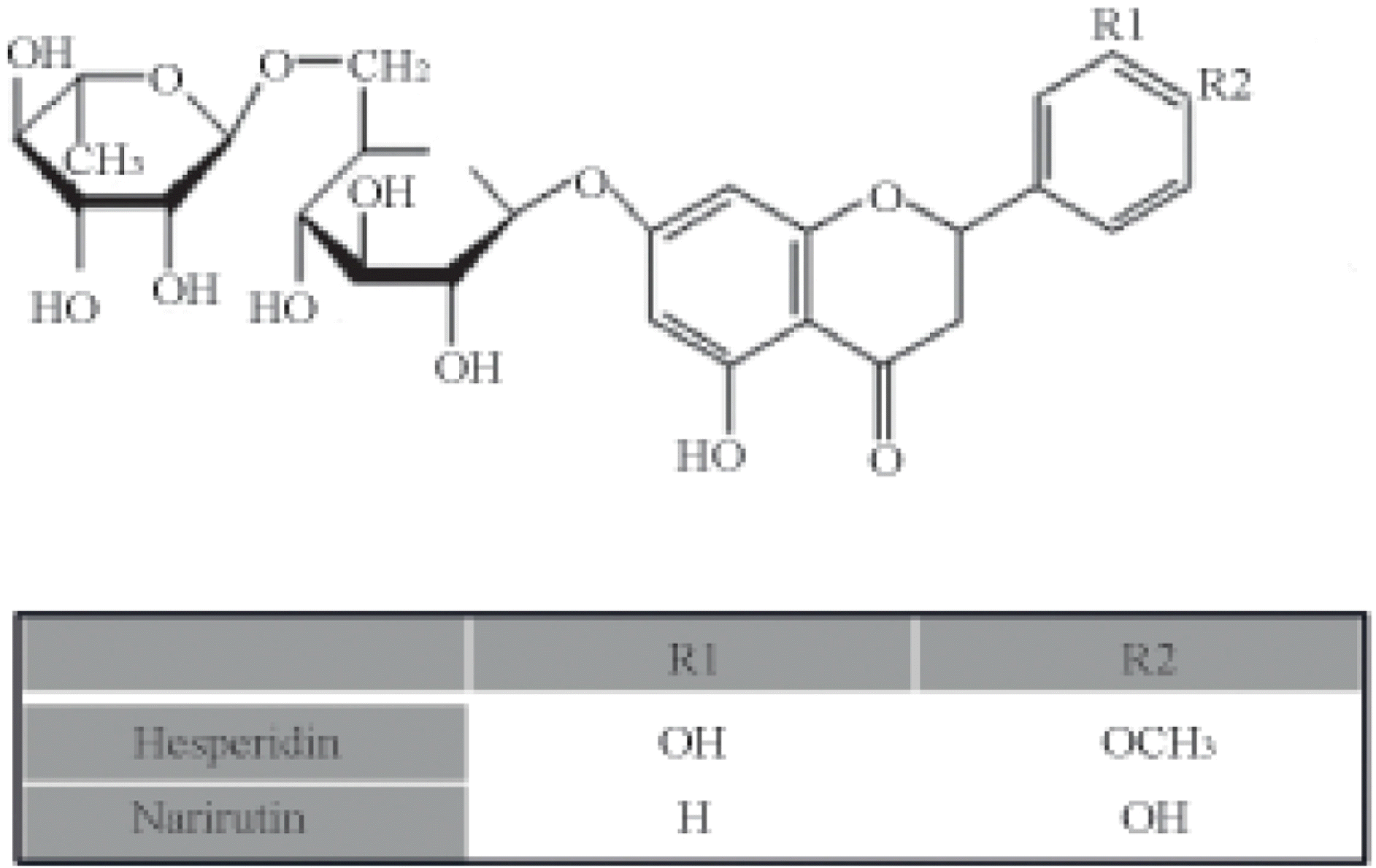
Abstract
Aqueous extraction of Citrus unshiu peels (AECUP) is mainly comprised with pro-angiogenichesperidin and narirutin. In this study, we report approaches to increasing the yields of extracted hesperidin and narirutinfrom Citrus unshiu peels using proper solvents. Significantly improved yields of both compounds were obtained using methanol and dimethyl sulfoxide (DMSO) compared to acetonitrile, ethyl acetate, ethanol, and isopropyl alcohol. Especially, effect of DMSO was by far the better of the two solvents in extraction of hesperidin. In addition, the DMSO extracted hesperidin significantly induced the pro-angiogenic effects of human umbilical vein endothelial cells (HUVECs) and markedly up-regulated phosphorylation of the ERK1/2 signaling pathway. These results demonstrate that pro-angiogenic inducer; hesperidin and narirutin can be simply, easily, and effectively extracted from Citrus unshiu peels.
Go to : 
References
(1). Choi I. Y.., Kim S. J.., Jeong H. J.., Park S. H.., Song Y. S.., Lee J. H.., Kang T. H.., Park J. H.., Hwang G. S.., Lee E. J.., Hong S. H.., Kim H. M.., Um J. Y.Mol. Cell. Biochem. 2007. 305:153–161.
(2). Jeong S. M.., Kim S. Y.., Kim D. R.., Jo S. C.., Nam K. C.., Ahn D. U.., Lee S. C. J.Agric. Food Chem. 2004. 52:3389–3393.
(3). Murakami A.., Nakamura Y.., Torikai K.., Tanaka T.., Koshiba T.., Koshimizu K.., Kuwahara S.., Takahashi Y.., Ogawa K.., Yano M.., Tokuda H.., Nishino H.., Mimaki Y.., Sashida Y.., Kitanaka S.., Ohigashi H.Cancer Res. 2000. 15:5059–5066.
(4). Kim D. K.., Lee K. T.., Eun J. S.., Zee O. P.., Lim J. P.., Eum S. S.., Kim S. H.., Shin T. Y.Arch. Pharm. Res. 1999. 22:642–645.
(5). Higashi-Okai K.., Kamimoto K.., Yoshioka A.., Okai Y.Phytother. Res. 2002. 16:781–784.
(6). Manthey J. A.., Grohmann K. J.Agric. Food Chem. 2001. 49:3268–3273.
(7). Chau C. F.., Huang Y. L. J.Agric. Food Chem. 2003. 51:2615–2618.
(8). Llorach R.., Espín J. C.., Tomás-Barberán F. A.., Ferreres F. J.Agric. Food Chem. 2003. 51:2181–2187.
(9). Lee J.., Yang D. S.., Han S. I.., Yun J. H.., Kim I. W.., Kim S. J.., Kim J. H. J.Med. Food. 2016. 19:569–577.
(10). Khurana R.., Simons M.., Martin J. F.., Zachary I. C.Circulation. 2005. 112:1813–1824.
(11). Folkman J.Nat. Med. 1995. 1:27–31.
(12). Redlitz A.., Daum G.., Sage E. H. J.Vasc. Res. 1999. 36:28–34.
(13). Teruyama K.., Abe M.., Nakano T.., Iwasaka-Yagi C.., Takahasi S.., Yamada S.., Sato Y. J.Cell. Physiol. 2001. 188:243–252.
(14). Bucar F.., Wube A.., Schmid M.Nat. Prod. Rep. 2013. 30:525–545.
(15). Lee J.., Han S. I.., Yun J. H.., Kim J. H.Tumour Biol. 2015. 36:9385–9393.
(16). Lee J.., Kim J. H.PLoS one. 2016. 11:, e0155264.
(17). Sanagi M. M.., Ling S. L.., Nasir Z.., Hermawan D.., Ibrahim W. A.., Abu Nami A. J.AOAC Int. 2009. 92:1833–1838.
(18). Anwar F.., Przybylski R.Acta. Sci. Pol. Technol. Aliment. 2012. 11:293–301.
(19). Jacob S. W.., Rosenbaum E. E.Headache. 1966. 6:127–136.
(20). Jacob S. W.., Wood D. C.Am. J. Surg. 1967. 114:414–426.
(21). Jacob S. W.Am. Surg. 1969. 35:564–573.
(22). Wood D. C.., Weber F. S.., Palmquist M. A. J.Pharmacol Exp. Ther. 1971. 177:520–527.
(23). Carmeliet P.Nature. 2005. 438:932–936.
(24). Dor Y.., Djonov V.., Abramovitch R.., Itin A.., Fishman G. I.., Carmeliet P.., Goelman G.., Keshet E.EMBO J. 2002. 21:1939–1947.
Go to : 
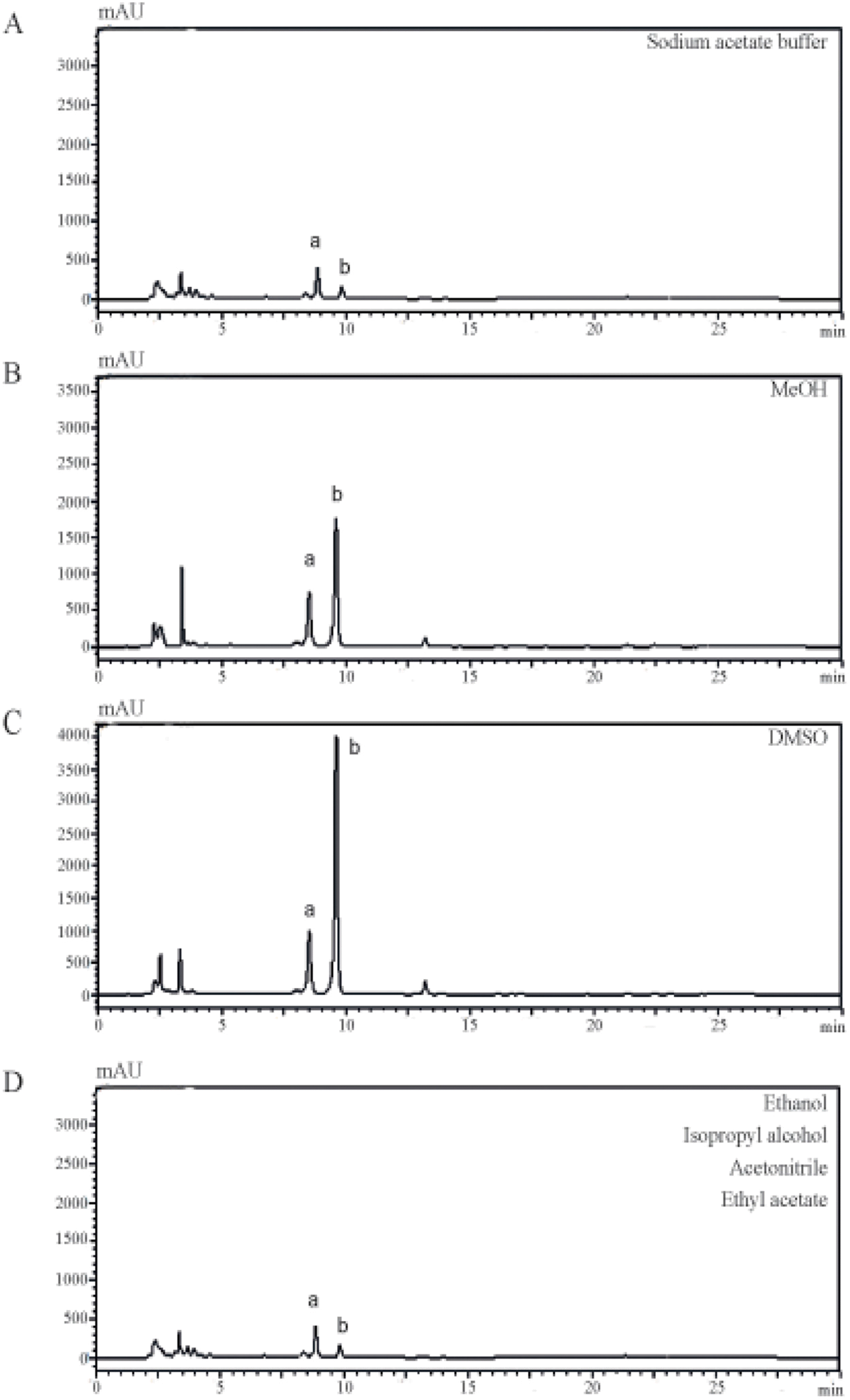 | Fig. 2.Characterization of constituent elements using different solvents. Citrus Unshiu peel was extracted with sodium acetate buffer (A), MeOH (B), or DMSO (C). The extracts were dissolved in methanol and were injected into a HPLC system at a column flow rate of 1 mL/min and analyzed (two major peaks of narirutin and hesperidin were designated a and b, respectively). |
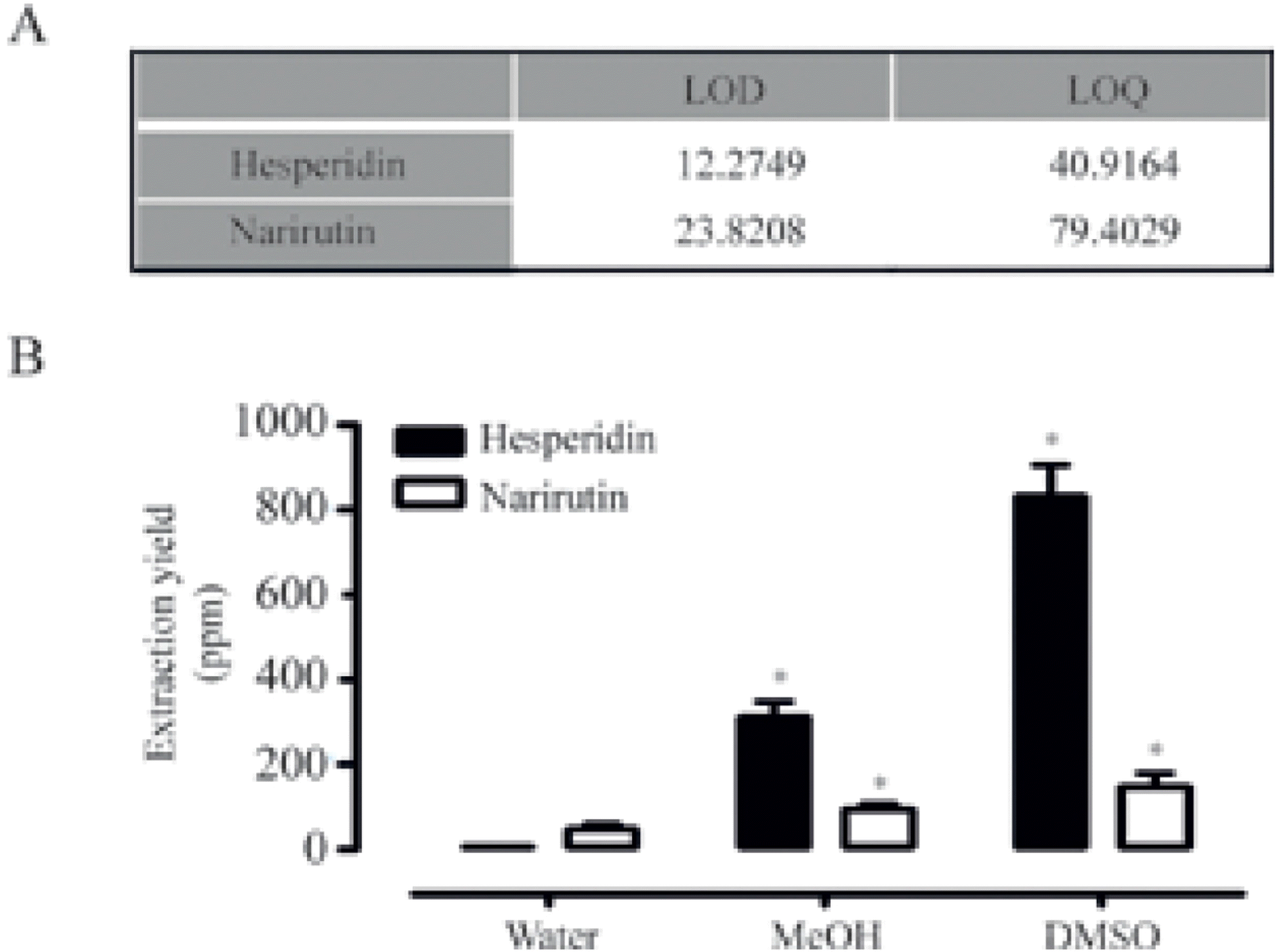 | Fig. 3.Quantification of extracted narirutin and hesperidin.(A) LOD and LOQ values of standard hesperidin and narirutin were calculated using HPLC chromatograms, respectively. (B) Extracted hesperidin and narirutin were quantified using HPLC analysis (data represent the percentage ± SD and are representative of three individual experiments, ∗p < 0.05). |
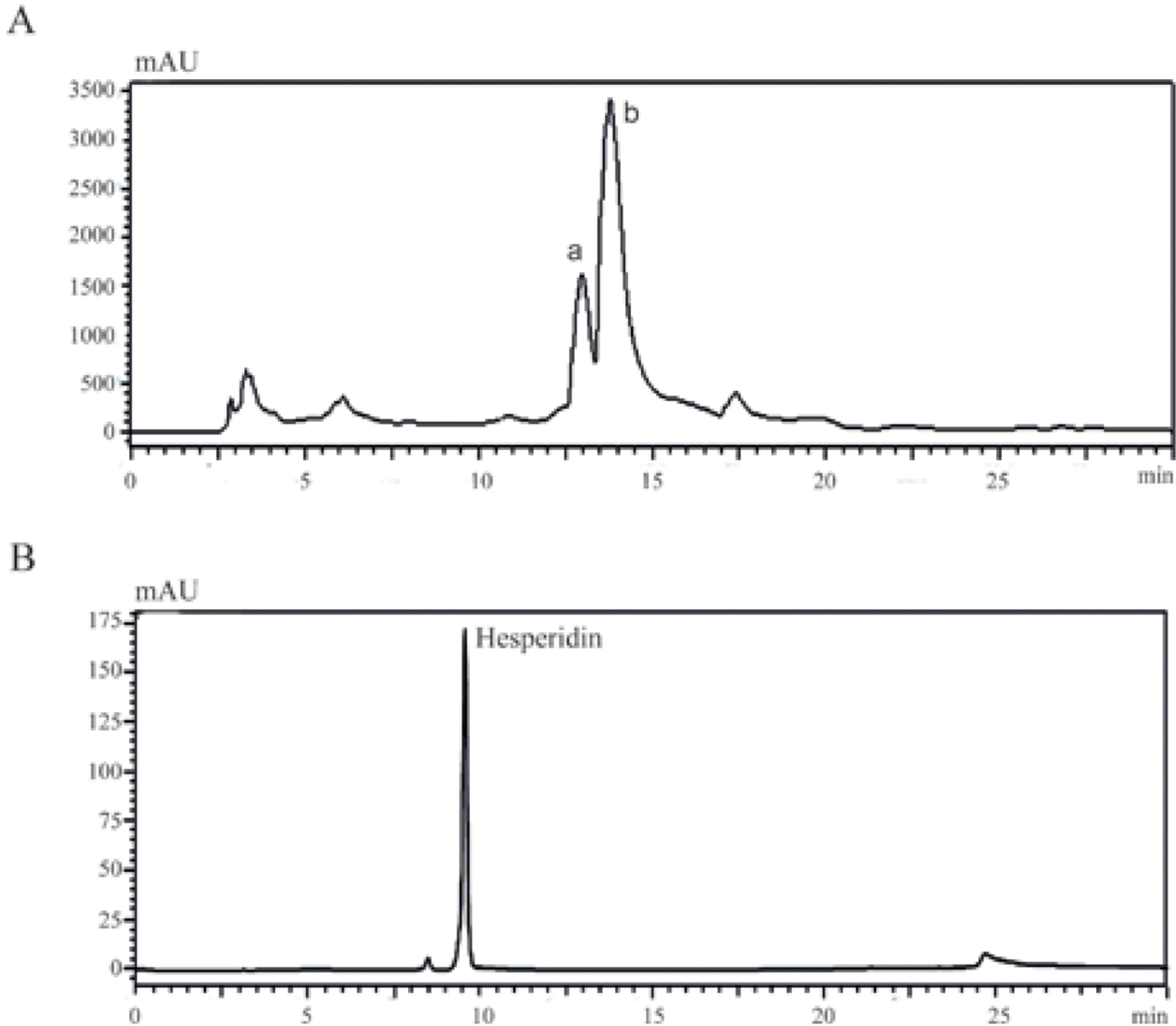 | Fig. 4.Purification of DMSO extracted hesperidin from Citrus Unshiu peel. (A) Extracted hesperidin was purified using a prep-LC system (two major peaks of narirutin and hesperidin were designated a and b, respectively). (B) Extracted hesperidin and standard hesperidin were dissolved in methanol and injected into the HPLC system at a column flow rate of 1 mL/min. |
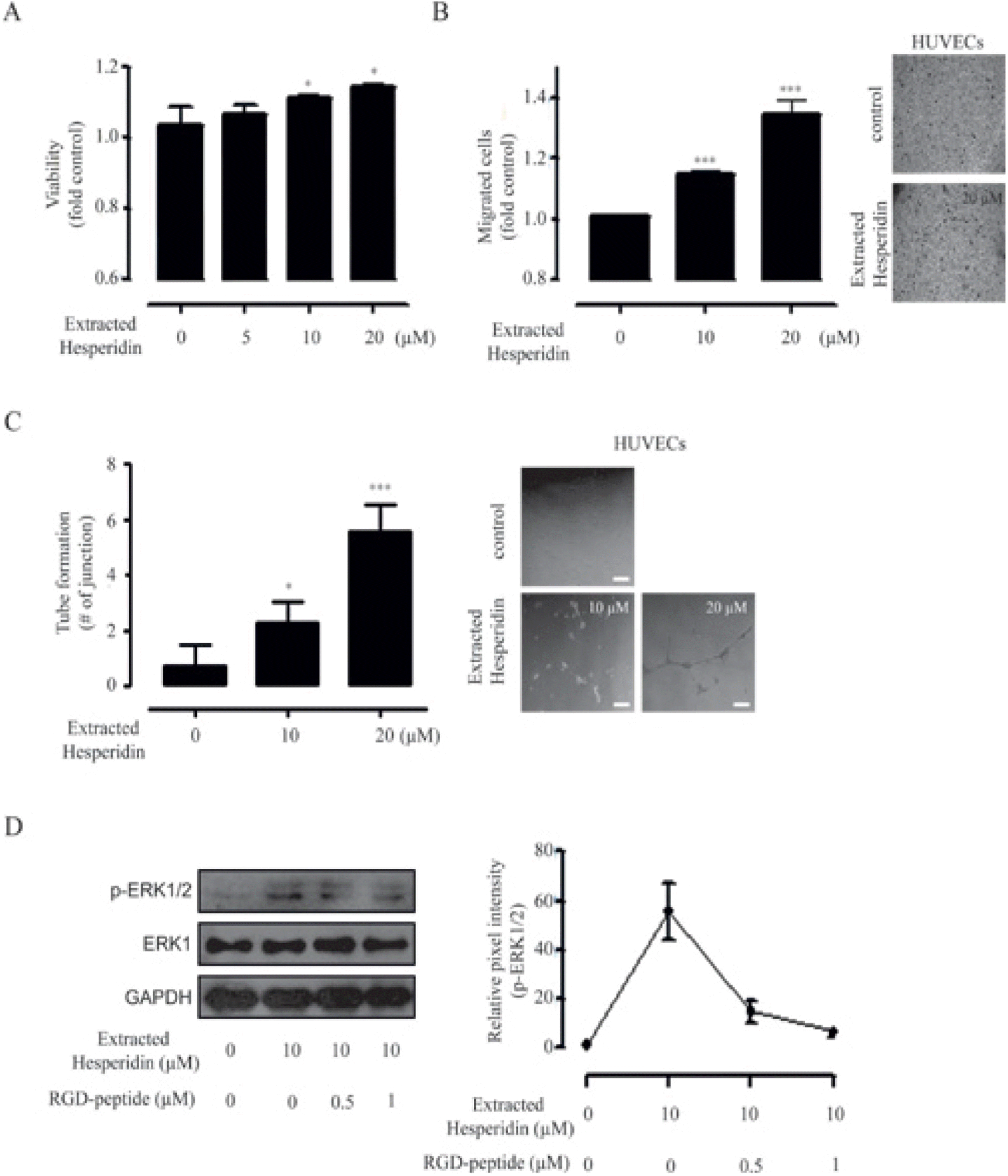 | Fig. 5.Pro-angiogenic effects of DMSO extracted hesperidin.(A) HUVECs were incubated with varying concentrations (0, 5, 10, and 20 µM) of DMSO extracted hesperidin for 72 h (data represent the percentage ± SD and are representative of three individual experiments, ∗p < 0.05, ∗∗p < 0.01). (B) HUVECs were incubated in the presence or absence of DMSO extracted hesperidin for 6 h using a Transwell migration assay (data represent the percentage ± SD and are representative of three individual experiments, ∗∗p < 0.01, ∗∗∗p <0.001). Representative image of a Transwell migration assay (scale bar=50µm). (C) Left, Capillary like structure (CLS) formation of HUVECs assayed after 16 h of incubation of cells in the presence or absence of DMSO extracted hesperidin(data represent the percentage ± SD and are representative of two individual experiments, ∗p < 0.05, ∗∗∗p < 0.001). Right, Representative image of tube-formation assay (scale bar = 50 µm).(D) Left, HUVECs were pre-incubated with different dose of RGD-peptide (0, 0.5, and 1 µM) for 30 min before DMSO extracted hesperidin (10 µM) were added for 60 min and cell lysates were subjected to immunoblot analysis using antibodies for p-ERK1/2 and ERK1. GAPDH was used as a loading control. Right, relative pixel intensities for p-ERK1/2 were measured using p-ERK1/2/GAPDH. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download