Abstract
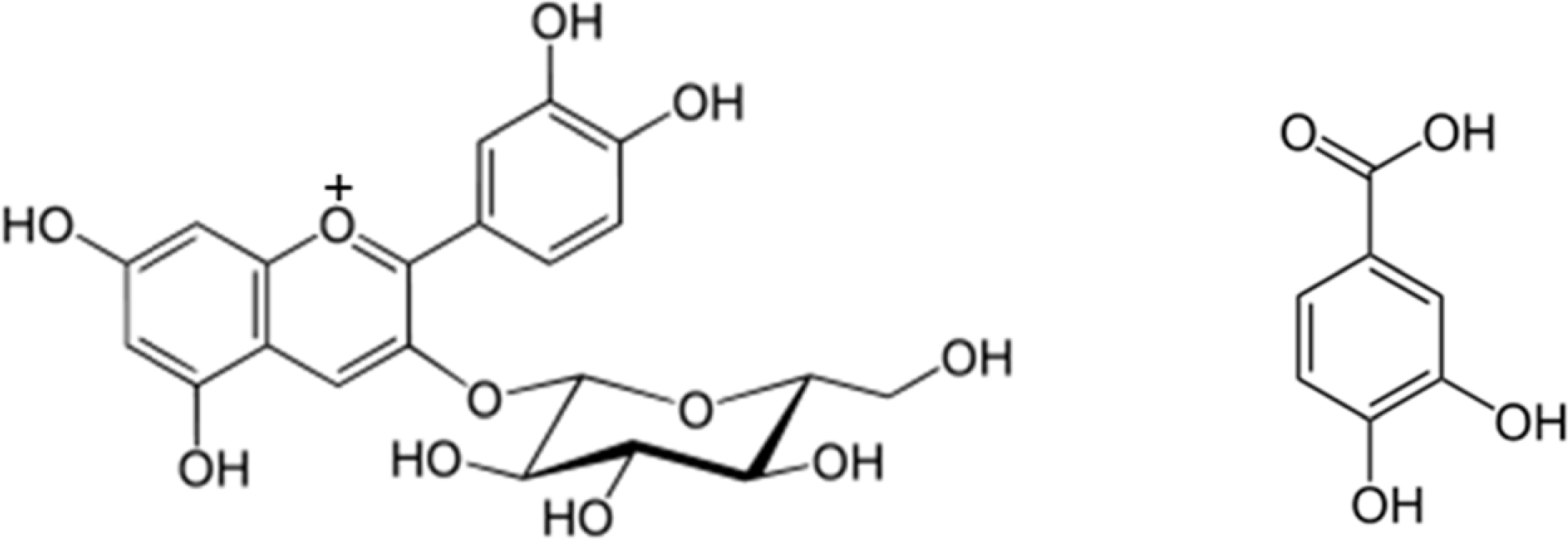
Cyanidin-3β-D-glycoside (C3G), which is widely distributed in herbal medicines and functional foods, exhibits anti-inflammatory, anti-oxidant, and anti-scratching behavioral effects. Orally administered C3G is metabolized to protocatechuic acid (PA) by gut microbiota. Therefore, we compared the anti-colitic effect of C3G to that of PA in mice with 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis. Orally administered C3G and PA preventively and curatively ameliorated TNBS-induced colitis parameters, including macroscopic colitis score, colon shortening, and increase of myeloperoxidase activity. Treatment with C3G or PA also inhibited the expression of cyclooxygenase-2, inducible NO synthatase, IL-1β, IL-6, and TNF-α and the activation of NF-κB in the colon of mice with TNBS-induced colitis. Furthermore, these also inhibited lipopolysaccharide-induced NF-κB activation and TNF-α expression in peritoneal macrophages. The anti-colitic effect of PA was more effective than C3G. Orally administered PA more potently attenuate colitis than C3G by inhibiting NF-κB activation and the anti-colitic efficacy of C3G may be dependent on the biotransformation of C3G to PA by gut microbiota.
References
(2). Han S. J.., Ryu S. N.., Trinh H. T.., Joh E. H.., Jang S. Y.., Han M. J.., Kim D. H. J.Food Sci. 2009. 74:H253–H258.
(3). de Ferrars R. M.., Czank C.., Zhang Q.., Botting N. P.., Kroon P. A.., Cassidy A.., Kay C. D.Br. J. Pharmacol. 2014. 171:3268–3282.
(4). Joseph S. V.., Edirisinghe I.., Burton-Freeman B. M. J.Agric. Food Chem. 2014. 62:3886–3903.
(5). Yang J.., Xiao Y. Y.Crit. Rev. Food Sci. Nutr. 2013. 53:1202–1225.
(6). Tsuda T.., Horio F.., Osawa T.Biofactors. 2000. 13:133–139.
(7). Min S.W.., Ryu S. N.., Kim D. H.Int. Immunopharmacol. 2010. 10:959–966.
(8). Vitaglione P.., Donnarumma G.., Napolitano A.., Galvano F.., Gallo A.., Scalfi L.., Fogliano V. J.Nutr. 2007. 137:2043–2048.
(9). Faria A.., Fernandes I.., Norberto S.., Mateus N.., Calhau C. J.Agric. Food Chem. 2014. 62:6898–6902.
(10). di Gesso J. L.., Kerr J. S.., Zhang Q.., Raheem S.., Yalamanchili S. K.., O'Hagan D.., Kay C. D.., O'Connell M. A.Mol. Nutr. Food Res. 2015. 59:1143–1154.
(11). Lee S. Y.., Jeong J. J.., Eun S. H.., Kim D. H.Eur. J. Pharmacol. 2015. 762:333–343.
(12). Ebert E. C.., Hagspiel K. D.Dig. Dis. Sci. 2011. 56:295–302.
(13). Wallace K. L.., Zheng L. B.., Kanazawa Y.., Shih D. Q.World J. Gastroenterol. 2014. 20:6–21.
(14). Laveti D.., Kumar M.., Hemalatha R.., Sistla R.., Naidu V. G.., Talla V.., Verma V.., Kaur N.., Nagpal R.Inflamm. Allergy Drug Targets. 2013. 12:349–361.
(15). Fairweather D.., Rose N. R.Lupus. 2005. 14:646–651.
(16). Dauphinee S. M.., Karsan A.Lab. Invest. 2006. 86:9–22.
(17). Perkins N. D.., Gilmore T. D.Cell Death Differ. 2006. 13:759–772.
(18). Tsuda T.., Horio F.., Osawa T. J.Nutr. Sci. Vitaminol. 2002. 48:305–310.
(19). Zhang Y.., Lian F.., Zhu Y.., Xia M.., Wang Q.., Ling W.., Wang X. D.Inflamm. Res. 2010. 59:723–730.
(20). Masella R.., Santangelo C.., D'Archivio M.., Li Volti G.., Giovannini C.., Galvano F.Curr. Med. Chem. 2012. 19:2901–2917.
Fig. 2.
Experimental protocol. (A) Preventative effect for TNBS-induced colitis in mice. (B) Curative effect for TNBS-induced colitis in mice.
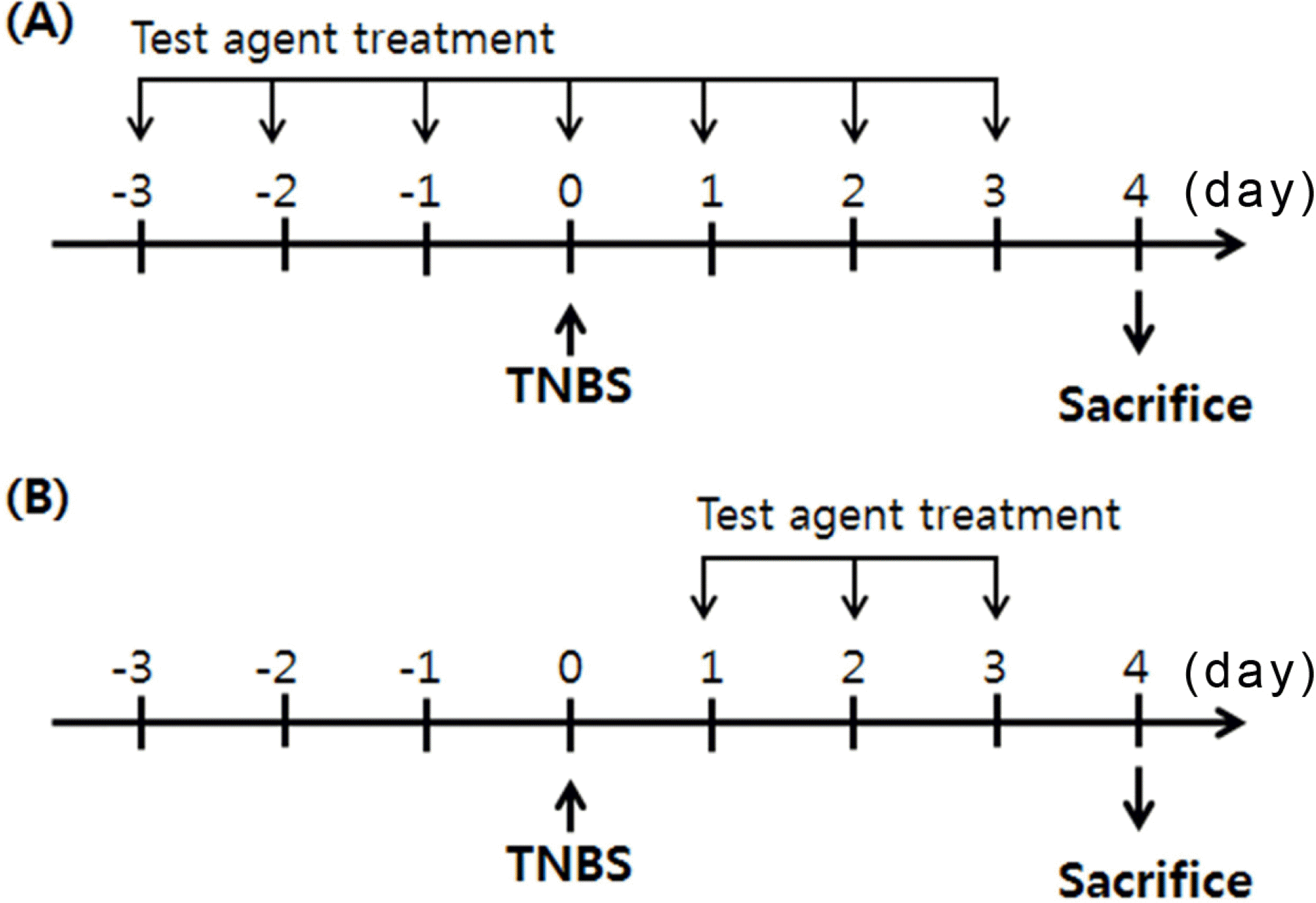
Fig. 3.
The preventive effects of C3G and PA on TNBS-induced colitis in mice. (A) Effects on body weight. (B) Effects on colon length. (C) Effects on macroscopic colitis score. (D) Effects on colonic MPO activity. (E) Effects on TNF-α, IL-1β, and IL-6 expression. (F) Effects on COX2 expression. TNBS, except normal group (NOR, treated with vehicle alone), was intrarectally administered. Test agents (CON, vehicle alone; C10, 10 mg/kg; C20, 20 mg/kg; PA, 10 mg/kg; SS, 20 mg/kg sulfasalazine) were orally administered from the 3rd day before TNBS treatment to the 3rd day after TNBS treatment and sacrificed 18 h after the final administration of test agents. All values are means (n = 10). #P < 0.05, significantly different vs. normal group; ∗P < 0.05, significantly different vs. control group.
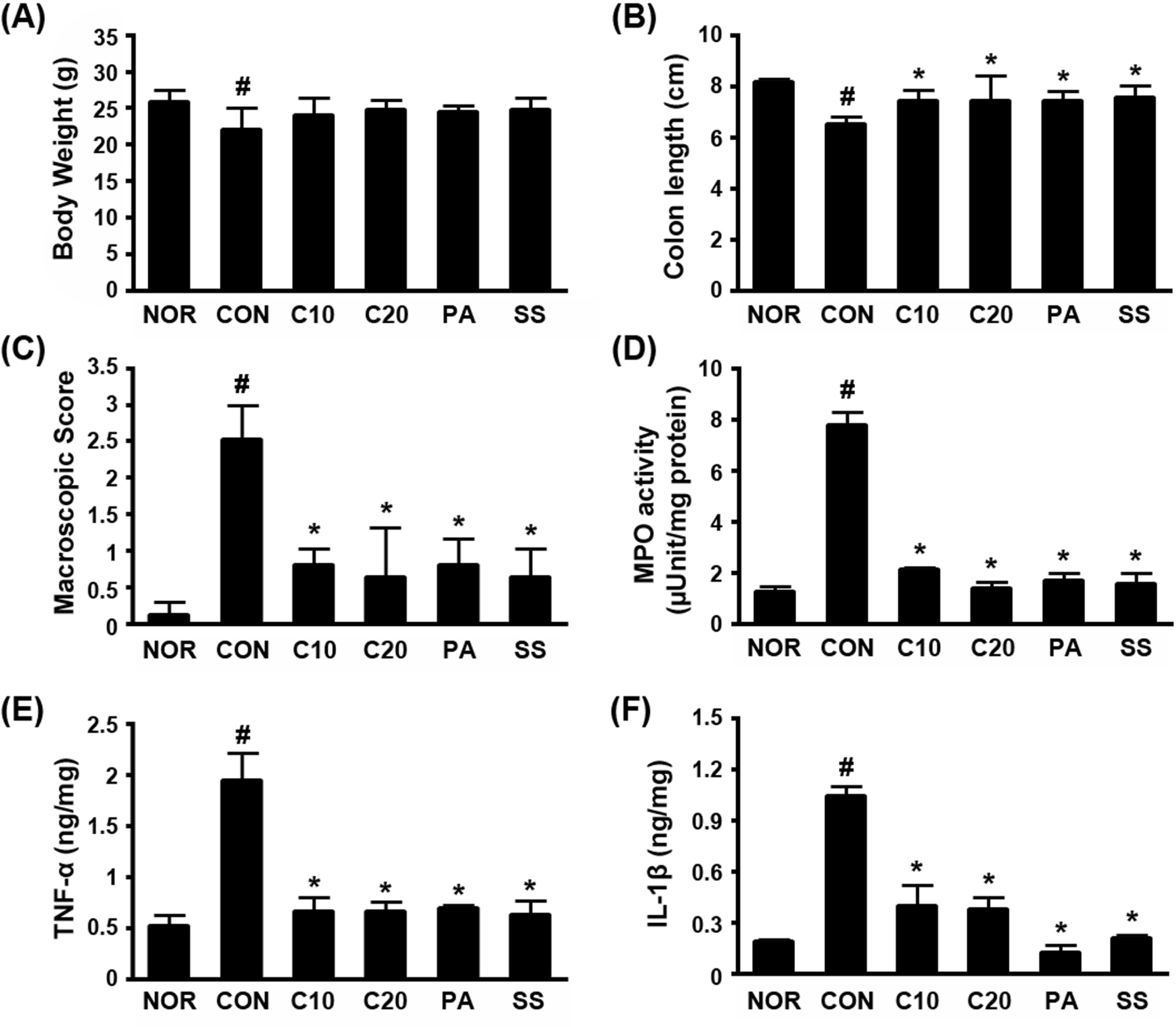
Fig. 4.
The curative effects of C3G and PA on TNBS-induced colitis in mice. (A) Effects on body weight. (B) Effects on colon length. (C) Effects on macroscopic colitis score. (D) Effects on colonic MPO activity. (E) Effects on TNF-α, IL-1β, and IL-6 expression. (F) Effects on COX2 and iNOS expression and NF-κB activation. TNBS, except normal group (NOR, treated with vehicle alone), was intrarectally administered. Test agents (CON, vehicle alone; C10, 10 mg/kg; C20, 20 mg/kg; PA, 10 mg/kg; SS, 20 mg/kg sulfasalazine) were orally administered for 3 days after TNBS treatment and sacrificed 18 h after the final administration of test agents. All values are means (n = 10). #P < 0.05, significantly different vs. normal group; ∗P < 0.05, significantly different vs. control group.
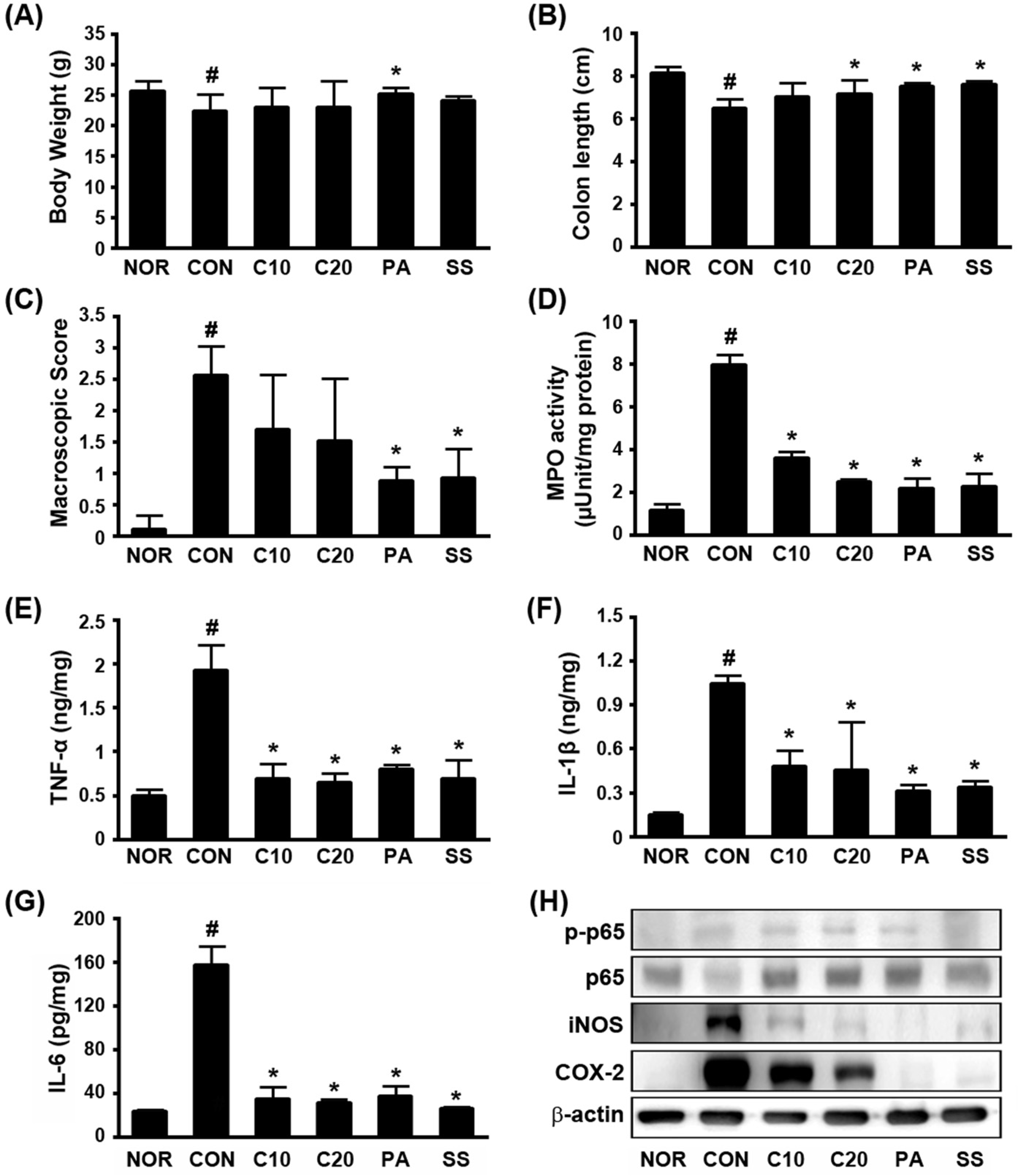
Fig. 5.
Effect of C3G and PA on NF-κB activation and TNF-α expression in LPS-stimulated peritoneal macrophages. Peritoneal macrophages (0.5 × 106 cells) were incubated with LPS in the absence or presence of C3G (C10, 10 μM; C20, 20 μM) or PA (P10, 10 μM) for 90 min (for NF-κB) or 20 h (for TNF-α). All data are shown as the mean ± S.D. (n = 3). #p < 0.05 vs. group treated without LPS and test agents. ∗p < 0.05 vs. group treated with LPS alone.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download