Abstract
Vitex trifolia L. has been used traditionally to treat various illnesses, such as inflammation, headache, migraine, and gastrointestinal infections. We analyzed and evaluated the composition of V. trifolia leaf oil. Based on the results, we isolated abietatriene from V. trifolia leaf oil and investigated the effect of V. trifolia leaf oil and its active compound abietatriene on melanogenesis in B16F10 melanoma cells. They significantly decreased melanin contents and melanogenic factors, such as tyrosinase, TRP-1, TRP-2, and MITF dose-dependently in both protein and mRNA levels. Protein and mRNA expressions were determined by Western blot analysis and quantitative real time RT-PCR. Findings indicate that V. trifolia leaf oil and abietatriene reduce melanogenesis by regulating the expression of melanogenic factors. These results suggest that V. trifolia leaf oil and abietatriene could comprise a useful therapeutic agent for treating hyperpigmentation and used as effective skin-whitening agents.
References
(1). Costin G. E.., Hearing V. J.FASEB J. 2007. 21:976–994.
(2). Lin J.Y.., Fisher D. E.Nature. 2007. 445:843–850.
(4). Tsukamoto K.., Jackson I. J.., Urabe K.., Montague P. M.., Hearing V. J.EMBO J. 1992. 11:519–526.
(5). Bentley N. J.., Eisen T.., Goding C. R.Mol. Cell. Biol. 1994. 14:7996–8006.
(6). Yasumoto K.., Yokoyama K.., Takahashi K.., Tomita Y.., Shibahara S. J.Biol. Chem. 1997. 272:503–509.
(7). Sugumaran M.Pigment Cell Res. 2002. 15:2–9.
(8). Chen J. S.., Wei C. I.., Marshall M. R. J.Agric. Food Chem. 1991. 39:1897–1901.
(9). Zhu W.., Gao J. J.Investig. Dermatol. Symp. Proc. 2008. 13:20–24.
(10). Kimura T.., Kimura T.Medicinal Plants of Japan in Color; Hoikusha Publishing: Osaka,. 1981. , p. 183.
(11). Ono M.., Sawamura H.., Ito Y.., Mizuki K.., Nohara T.Phytochemistry. 2000. 8:873–877.
(12). Park J. H.., Lee C. K.The Encyclopedia of Medicinal Plants; Shinilbooks: Korea,. 2000. 183–184:284–289.
(13). Kim C. M.., Lee Y. J.., Kim I. L.., Shin J. H.., Kim Y. I.Coloured Illustrations dor Discrimination of Herbal Medicine; Academybooks: Korea:. 2015. , p. 278.
(14). Huang M. Y.., Zhong L. J.., Xie J. M.., Wang F.., Zhang H. Y.Helv. Chim. Acta. 2013. 96:2040–2045.
(15). Institute of Materia Medica. Chinese Academy of Medical Sciences, Chinese Materia Medica. People's Medical Publishing House;Beijing: 1984. Vol. 3:, p. p. 679.
(16). Lee M. K.., Kim D. H.., Park T. S.., Son J. H. J.Appl. Biol. Chem. 2015. 58:125–129.
(17). Ramesh P.., Nair A. G. R.., Subramanian S. S.Fitoterapia. 1986. LVII(4):282–283.
(18). Thein K.., Myint W.., Myint M. M.., Aung S. P.., Khin M.., Than A.., Bwin M.Pharm. Biol. 1995. 33:330–333.
(19). Hernández M. M.., Heraso C.., Villarreal M. L.., Vargas-Arispuro I.., Aranda E. J.Ethnopharmacol. 1999. 67:37–44.
(20). Hossain M. M.., Paul N.., Sohrab M. H.., Rahman E.., Rashid M. A.Fitoterapia. 2001. 72:695–697.
(21). Zeng X.., Fang Z.., Wu Y.., Zhang H.Chung Kuo Chung Yao Tsa Chih. 1996. 21:167–168.
(22). Ramesh P.., Nair A. G. R.Fitoterapia. 1986. 57:282.
(23). Nair A. G. R.., Ramesh P.., Subramanian S. S.Curr. Sci. 1975. 44:214–216.
(24). Vedantham T. N. C.., Subramanian S. S.Indian J. Pharmacol. 1976. 38:13–24.
(25). Pan J. G.., Xu Z. L.., Fan J. F.Chung Kuo Chung Yao Tsa Chih. 1989.
(26). Zai-bo Y.., Chao Z.Journal of Henan University(Medical Science). 2006. 4:4.
(27). Suksamrarn A.., Werawattanametin K.., Brophy J. J.Flavour Frag. J. 1991. 6:97–99.
(28). Hill S. E.., Buffey J.., Thody A. J.., Oliver I.., Bleehen S. S.., Mac Neil S.Pigment Cell Res. 1989. 2:161–166.
(29). Livak K. J.., Schmittgen T. D.Methods. 2001. 25:402–408.
(30). Zi J.., Peters R. J.Org. Biomol. Chem. 2013. 11:7650–7652.
(31). Bhar S. S.., Ramana M. M. V. J.Org. Chem. 2004. 69:8935–8937.
(32). Yamaguchi Y.., Beer J. Z.., Hearing V. J.Arch. Dermatol. Res. 2008. 300:43–50.
Fig. 2.
The cell viability of V. trifolia leaf oil and abietatriene. B16F10 melanoma cells were treated with V. trifolia leaf oil and abietatriene (1 – 100 μg/mL) for 48 h. Cell viability was measured by CCK-8 assay. Data are expressed as mean ± SD of three independent experiments. ∗p < 0.05, ∗∗p < 0.01 compared with DMSO control (−).
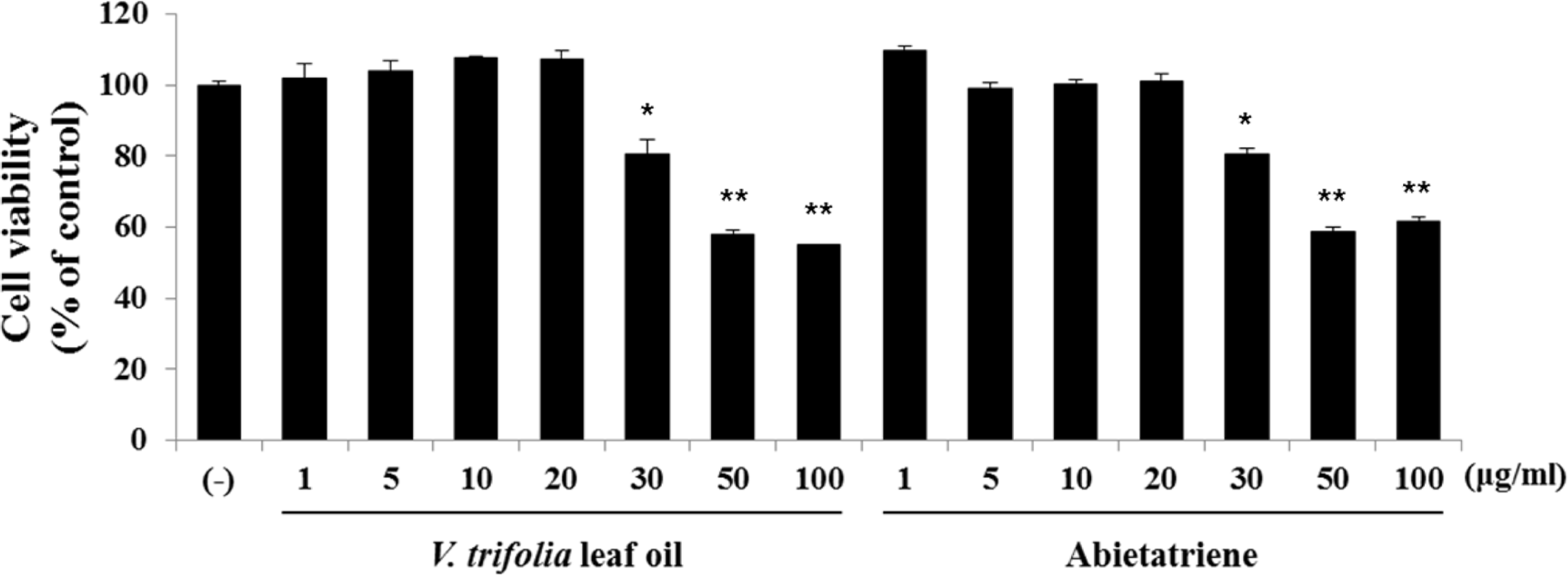
Fig. 3.
Effect of V. trifolia leaf oil fractions 1 – 8 on melanin content. B16F10 cells were treated with V. trifolia leaf oil fractions 1 – 8, V. trifolia leaf oil and abietatriene or arbutin for 48 h. (A) Melanin content of V. trifolia leaf oil fractions 1 – 8 (10, 20 μg/mL). (B) Melanin content of V. trifolia leaf oil fractions 1 – 8, V. trifolia leaf oil and abietatriene 20 μg/mL or arbutin 100 μg/mL. Melanin content was determined by spectrophotometry. Data are expressed as % of DMSO control (−).
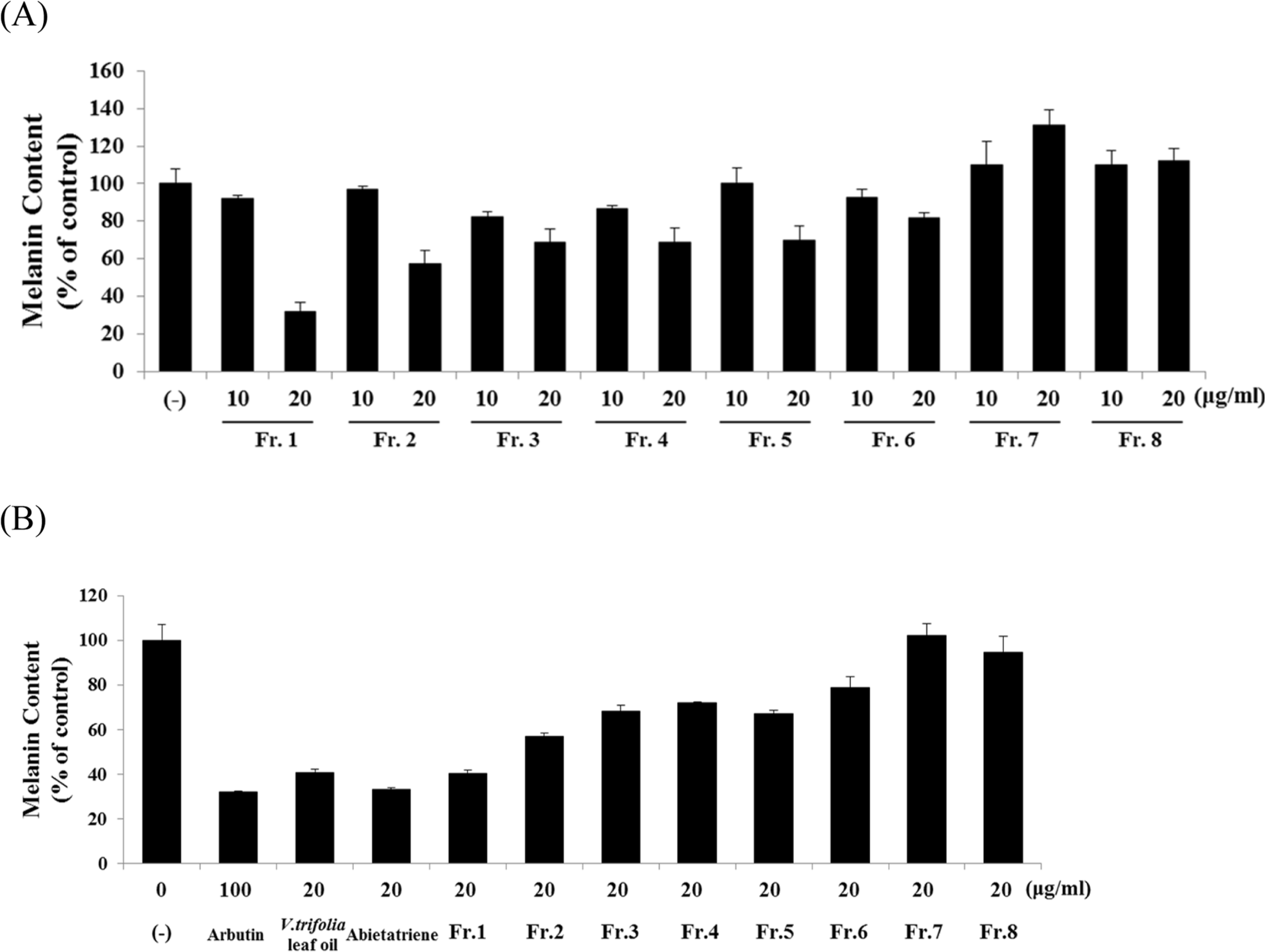
Fig. 4.
Effect of V. trifolia leaf oil and abietatriene on melanin content. B16F10 cells were treated with V. trifolia leaf oil and abietatriene (1 – 20 μg/mL) or arbutin 100 μg/mL for 48 h. Melanin content was determined by spectrophotometry. Data are expressed as % of control (DMSO) and expressed as mean ± SD of three independent experiments. ∗p < 0.05, ∗∗p < 0.01 compared with DMSO control (−).
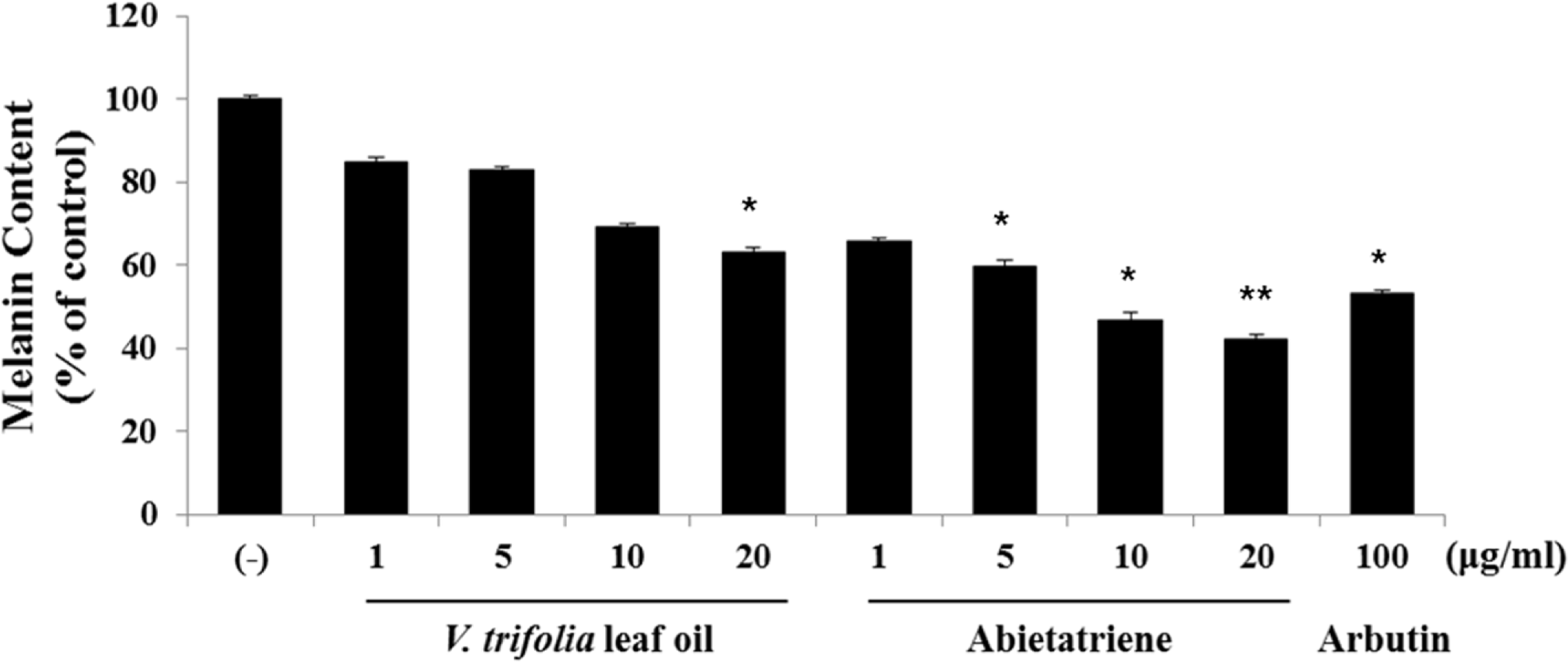
Fig. 5.
Effect of V. trifolia leaf oil and abietatriene on the protein expression of melanogenic factors. B16F10 cells were treated with V. trifolia leaf oil and abietatriene (1, 10, and 20 μg/mL) and incubated for 48 h. Cell lysates were evaluated by Western blot with antibodies against tyrosinase, TRP-1, TRP-2, and MITF. Equal protein loading was confirmed by staining with antibodies against â-actin. (A) The protein expression of melanogenic proteins. (B) Relative protein expression of each melanogenic proteins. Band intensity was quantified using ImageJ software. The results are expressed as % of control and each column represents the mean ± SD of three independent experiments. ∗p < 0.05, ∗∗p < 0.01 compared with DMSO control (−).
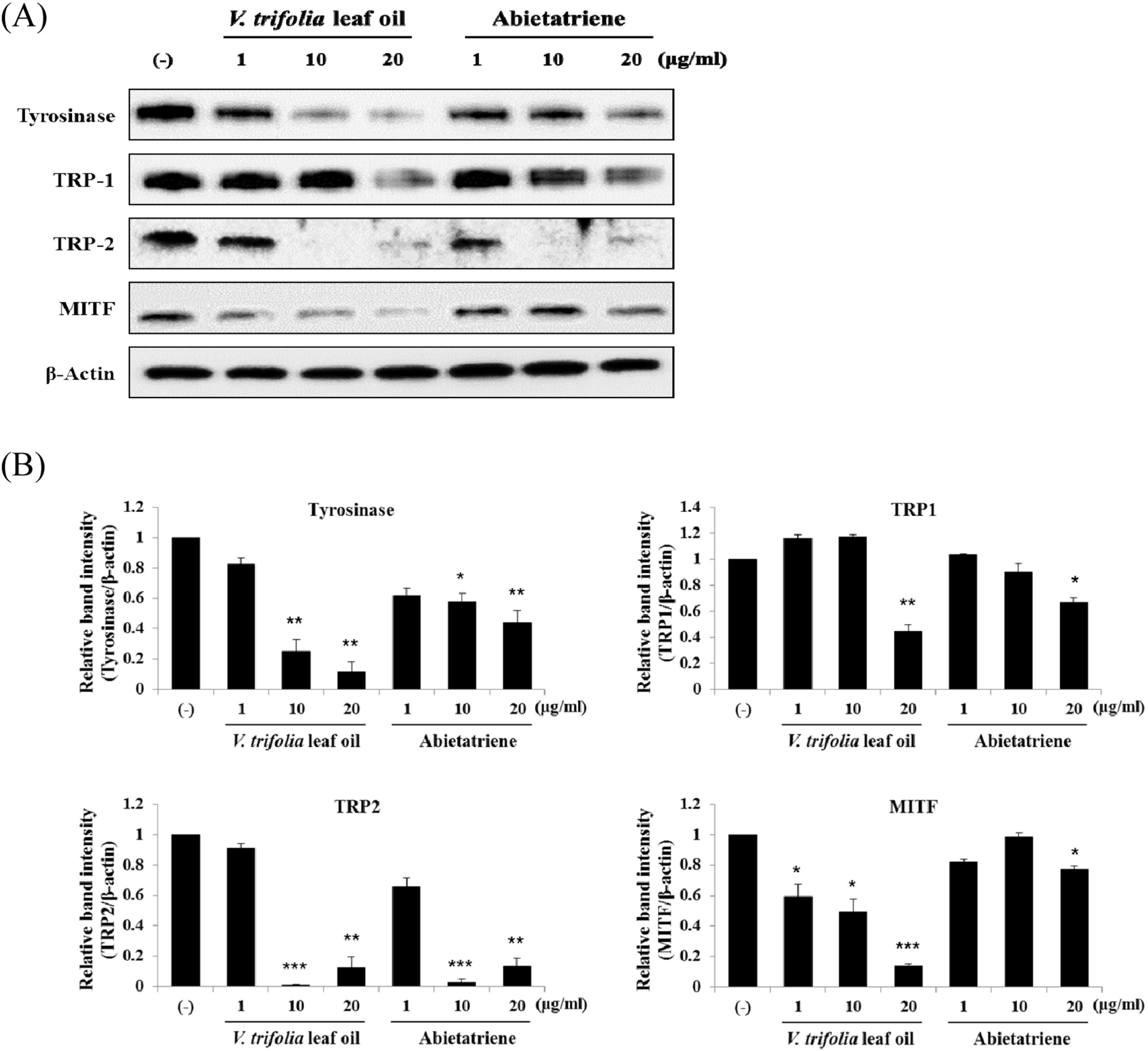
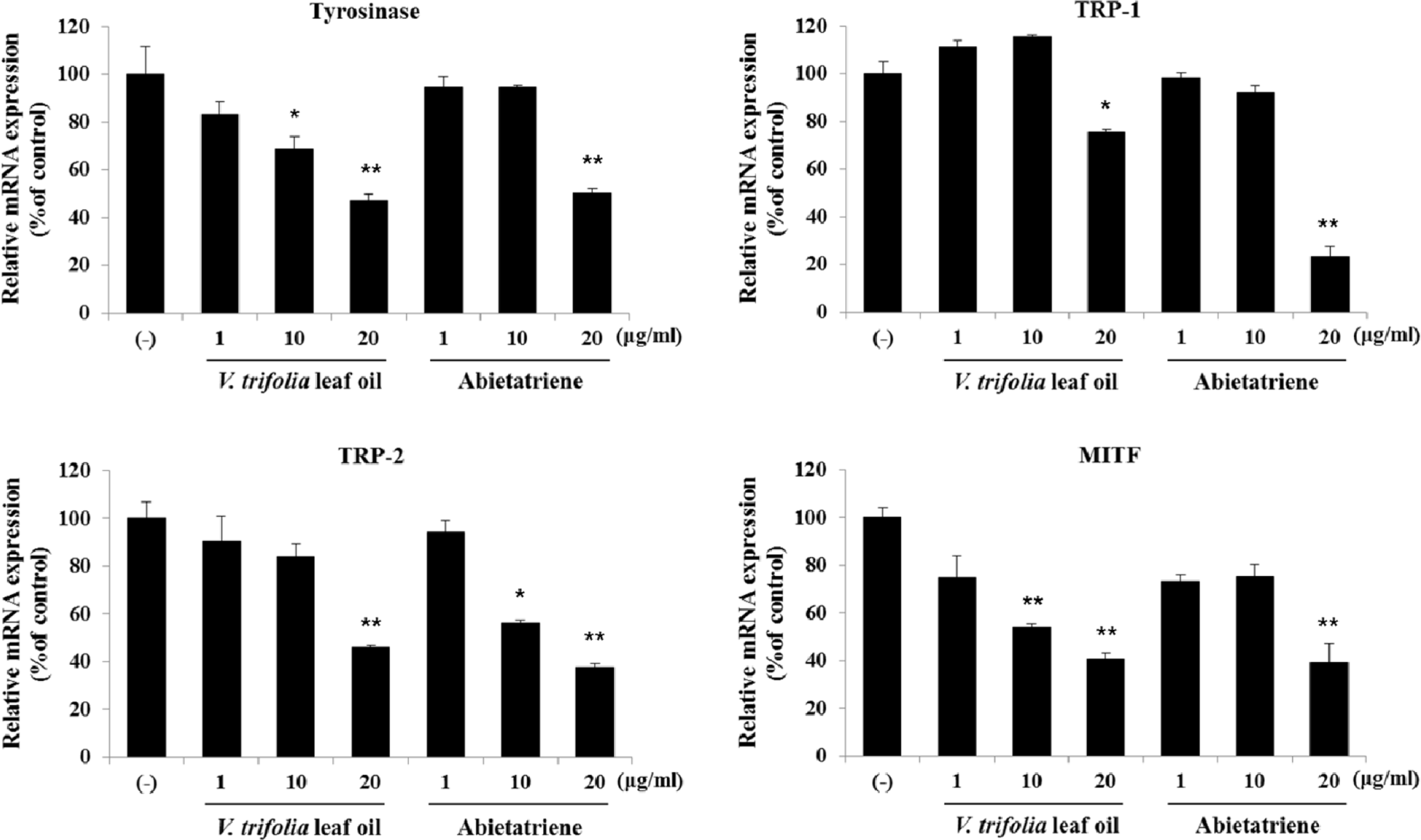
Fig. 6.
Effect of V. trifolia leaf oil and abietatriene on the mRNA expression of melanogenic factors. B16F10 cells were treated with V. trifolia leaf oil and abietatriene (1, 10 and 20 μg/mL) and incubated for 24 h. Cell lysates were evaluated by qPCR with Taqman probe against tyrosinase, TRP-1, TRP-2, and MITF. Equal mRNA loading was confirmed by GAPDH. The results are expressed as % of control, the mean ± SD of three independent experiments. ∗p < 0.05, ∗∗p < 0.01 compared with DMSO control (−).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download