Abstract
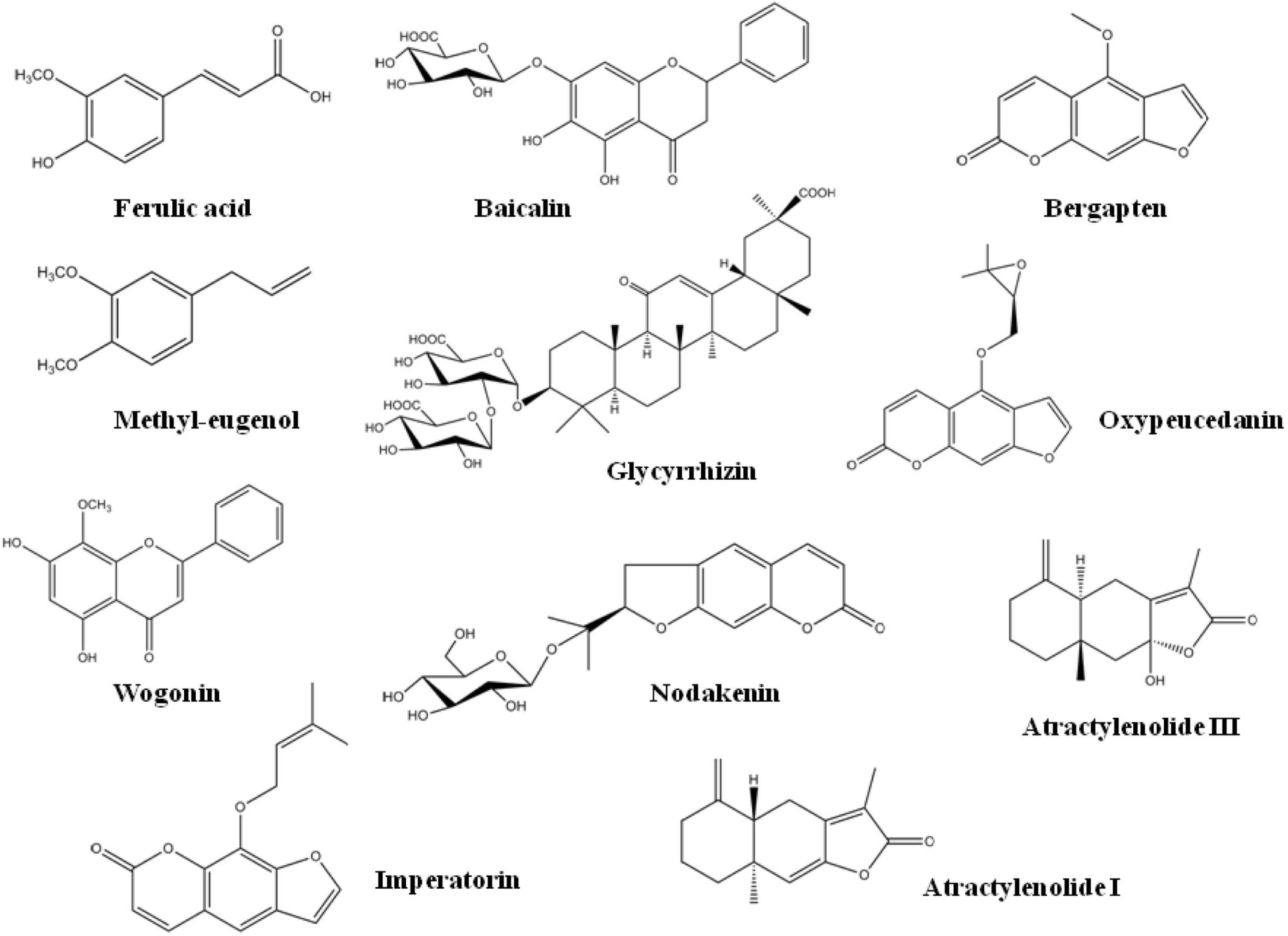
Gumiganghwal-tang has been used for the treatment of common cold for a long-time. We developed an accurate and sensitive high performance liquid chromatography-diode array detection (HPLC-DAD) and electrospray ionization mass spectrometry method for the simultaneous determination of ferulic acid, baicalin, bergapten, methyl eugenol, glycyrrhizin, oxypeucedanin, wogonin, nodakenin, atractylenolide III, imperatorin, and atractylenolide I in Gumiganghwal-tang samples. The analytes were separated on a Shiseido C18 column (5 µm, 4.6 mm I.D. × 250 mm) with gradient elution with acetonitrile and 0.1% trifluoroacetic acid. Eleven compounds were quantitatively determined by HPLC-DAD and identified by LC-MS data. We also validated this method. The calibration curves of all the compounds showed good linear regression. The limits of detection and the limits of quantification ranged from 0.04 to 0.63 and from 0.12 to 1.92µg/mL, respectively. The relative standard deviation values of intra- and inter-days of this method represented less than 2.9%. The recoveries were found to be in the range of 90.06–107.66%. The developed method has been successfully applied to the analysis of Gumiganghwal-tang samples. The established HPLC method could be used to quality control of Gumiganghwal-tang.
References
(1). Jiang W. Y.Trends Pharmacol. Sci. 2005. 26:558–563.
(2). Liang Y. Z.., Xie P. J.Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004. 812:53–70.
(3). Bent S. J.Gen. Intern. Med. 2008. 23:854–859.
(4). Firenzuoli F.., Gori L.Evid. Based Complement. Alternat. Med. 2007. 4:37–40.
(5). Moon Y. H.., Go J. J.., Park J. Y.Kor. J. Pharmacogn. 1999. 30:18–24.
(6). Kim S. J.., Jeong H. J.., Moon P. D.., Lee K. M.., Lee H. B.., Jung H. J.., Jung S. K.., Rhee H. K.., Yang D. C.., Hong S. H.., Kim H. M.Biol. Pharm. Bull. 2005. 28:233–237.
(7). Lee M. Y.., Seo C. S.., Shin I. S.., Ha H.., Kim J. H.., Cho J. W.., Huh J. I.., Shin H. K.Regul. Toxicol. Pharmacol. 2012. 62:553–560.
(8). Jung H. W.., Mahesh R.., Park J. H.., Boo Y. C.., Park K. M.., Park Y. K.Int. Immunopharmacol. 2010. 10:155–162.
(9). Jung H. W.., Jung J. K.., Park Y. K.AsianPac. J. Allergy Immunol. 2011. 29:338–348.
(10). Kang T. J.., Lee S. Y.., Singh R. P.., Agarwal R.., Yim D. S.Acta. Oncol. 2009. 48:895–900.
(11). Okuyama E.., Hasegawa T.., Matsushita T.., Fujimoto H.., Ishibashi M.., Yamazaki M.Chem. Pharm. Bull. 2001. 49:154–160.
(12). Chin Y. W.., Jung Y. H.., Chae H. S.., Yoon K. D.., Kim J.Bull. Korean Chem. Soc. 2011. 32:2132–2134.
(13). Li S.., Han Q.., Qiao C.., Song J.., Lung Cheng C.., Xu H.Chin. Med. 2008. 3:DOI: 10.1186/1749-8546-3-7.
(14). Choi D. W.., Kim J. H.., Cho S. Y.., Kim D. H.., Chang S. Y.Toxicology. 2002. 181–182:581–586.
(15). Lin S. J.., Tseng H. H.., Wen K. C.., Suen T. T. J.Chromatogr. A. 1996. 730:17–23.
(16). Liu Q.., Zhao J.., Yan L.., Yi J.., Song J.Zhongguo Zhong Yao Za Zhi. 2010. 35:708–710.
(17). Zuo F.., Zhou Z. M.., Liu M. L.Biol. Pharm. Bull. 2001. 24:693–697.
(18). Jiang P.., Shi R.., Wang Q.., Ma Y. M.., Cui H. Y.., Liu P.., Liu C. H.Biomed. Chromatogr. 2013. 27:874–881.
(19). Samanidou V.., Tsagiannidis A.., Sarakatsianos I. J.Sep. Sci. 2012. 35:608–615.
(20). Sun Q.., Chang L.., Ren Y.., Cao L.., Sun Y.., Du Y.., Shi X.., Wang Q.., Zhang L. J.Sep. Sci. 2012. 35:2897–2907.
(21). Weon J. B.., Yang H. J.., Ma J. Y.., Ma C. J. J.Nat. Med. 2012. 66:510–515.
(22). Shin I. S.., Seo C. S.., Lee M. Y.., Ha H. K.., Huh J. I.., Shin H. K. J.Ethnopharmacol. 2012. 141:350–356.
Fig. 3.
HPLC chromatogram of standard compounds mixture (A) and Gumiganghwal -tang sample (B). Peaks: (1) ferulic acid, (2) nodakenin, (3) baicalin, (4) bergapten, (5) glycyrrhizin, (6) oxypeucedanin, (7) wogonin, (8) methyl eugenol, (9) atractylenolide III, (10) imperatorin and (11) atractylenolide I.
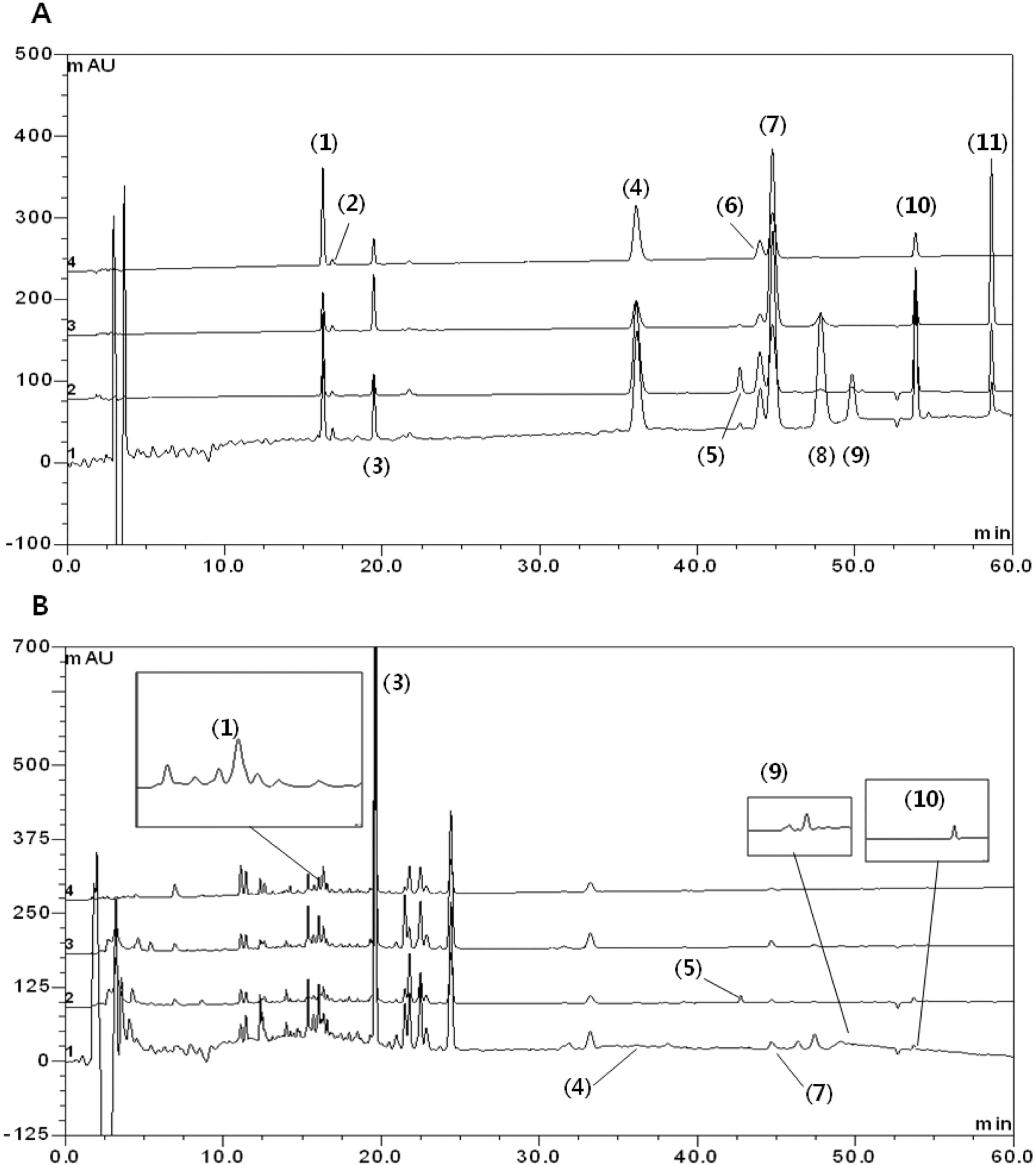
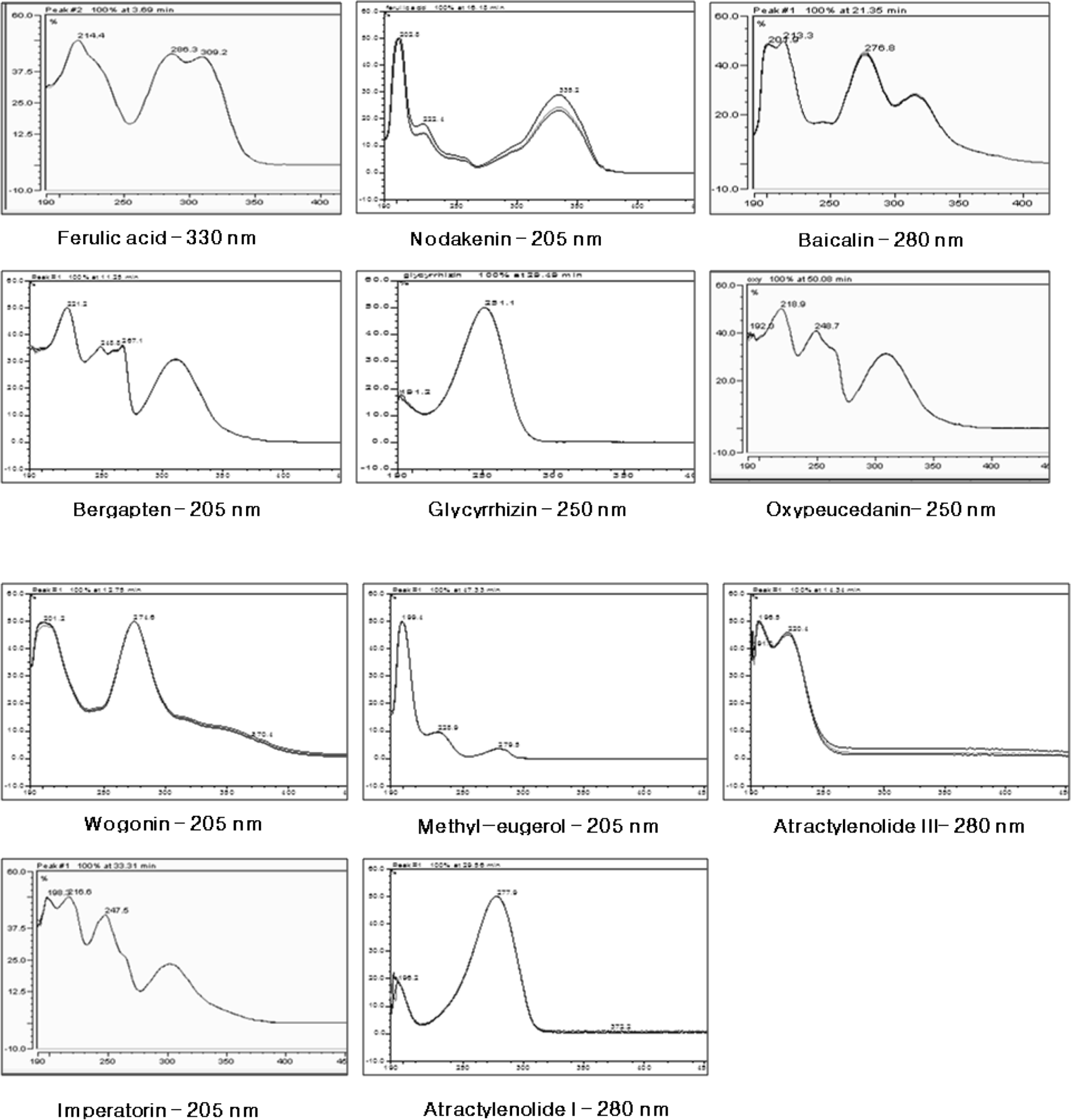
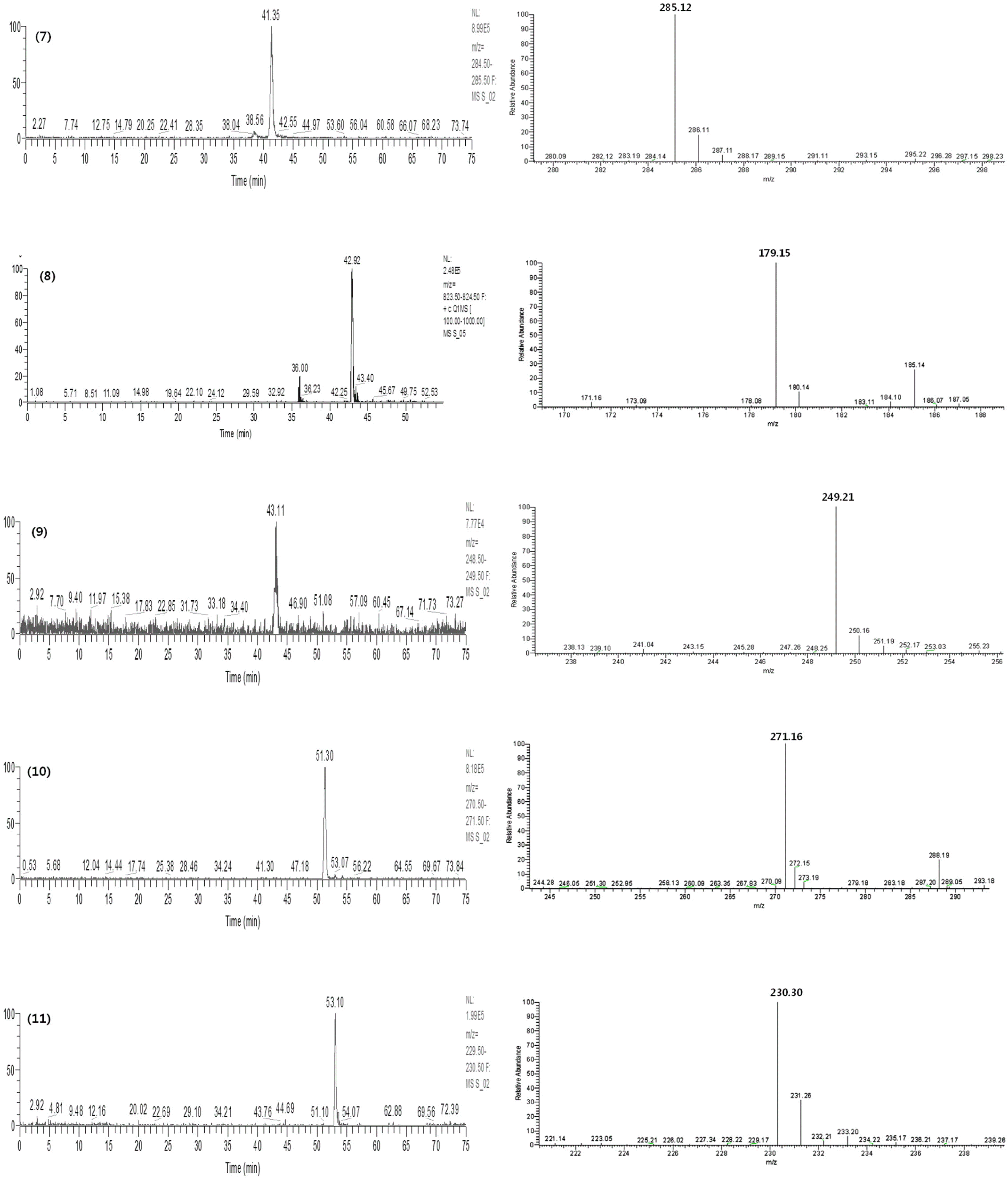
Fig. 4.
SIM chromatograms and product ion scan spectra of the 11 marker compounds of Gumiganghwal-tang. Numbers: (1) ferulic acid, (2) nodakenin, (3) baicalin, (4) bergapten, (5) glycyrrhizin, (6) oxypeucedanin, (7) wogonin, (8) methyl eugenol, (9) atractylenolide III, (10) imperatorin and (11) atractylenolide I.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download