Abstract
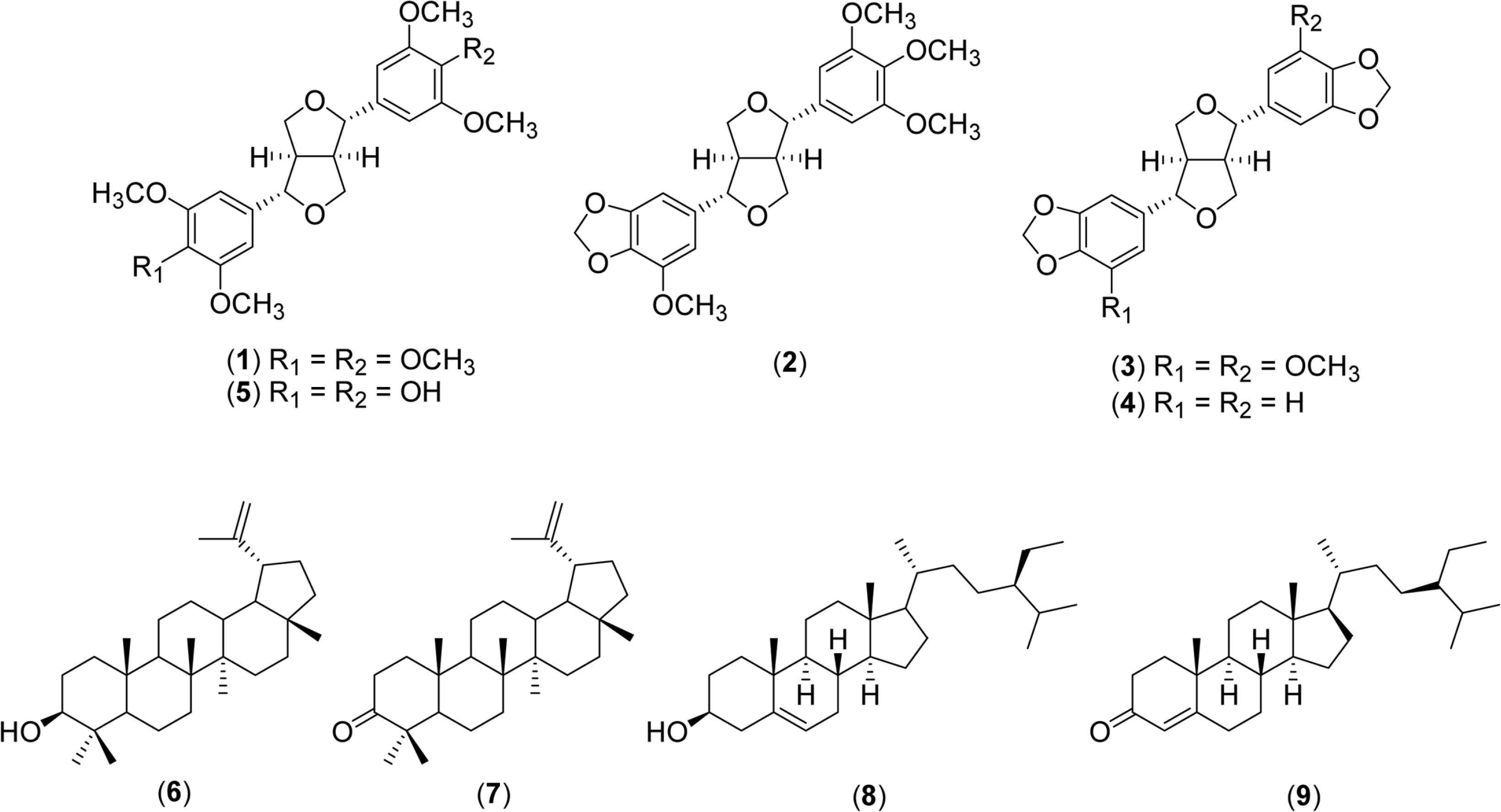
Phytochemical investigation from the stem bark of Beilschmiedia pulverulenta resulted in the isolation of five lignans, (+)-yangambin (1), (+)-sesartemin (2), (+)-excelsin (3), (+)-sesamin (4), and (+)-syringaresinol (5), together with lupeol (6), lupenone (7), β-sitosterol (8), and β-sitostenone (9). Their structures were established by the analysis of their spectroscopic (1D and 2D NMR) and spectrometric (MS) data, as well as by comparison with those reported in the literature. The isolated lignans were tested for their anticholinesterase (AChE: acetylcholine esterase and BChE: butyryl cholineesterase) and anti-inflammatory (COX-2: cyclooxygenase-2 and LOX: lipoxygenase) activities. All the isolated lignans (1 – 5) exhibited significant inhibition activities in AChE/BChE and COX-2/LOX assays with IC50 values ranging from 168.8 – 504.2 µM and 21.0 – 59.4 µM, respectively.
Go to : 
References
(1). Perry E. K.., Tomlinson B. E.., Blessed G.., Bergmann K.., Gibson P. H.., Perry R. H.Br. Med. J. 1978. 6150:1457–1459.
(2). Mehta M.., Adem A.., Sabbagh M.Int. J. Alzheimers Dis. 2012. , 10.1155/2012/728983.
(3). Borovikova L. V.., Ivanova S.., Nardi D.., Zhang M.., Yang H.., Ombrellino M.., Tracey K. J.Auton. Neurosci. 2000. 85:141–147.
(4). Tabet N.Age Ageing. 2006. 35:336–338.
(5). Banfield J. E.., Black D. S. C.., Collins D. J.., Hyland B. P. M.., Lee J. J.., Pranowo S. R.Aust. J. Chem. 1994. 47:587–607.
(6). Chen J. J.., Chou E. T.., Peng C. F.., Chen I. S.., Yang S. Z.., Huang H. Y.Planta Med. 2007. 73:567–571.
(7). Salleh W. M. N. H. W.., Ahmad F.., Yen K. H.Arch. Pharm. Res. 2015. 38:485–493.
(8). Lenta B. N.., Tantangmo F.., Devkota K. P.., Wansi J. D.., Chouna J. R.., Soh R. C. F.., Neumann B.., Stammler H. G.., Tsamo E.., Sewald N. J.Nat. Prod. 2009. 72:2130–2134.
(9). Chouna J. R.., Nkeng-Efouet P. A.., Lenta B. N.., Wansi J. D.., Kimbu S. F.., Sewald N.Phytochem. Lett. 2010. 3:13–16.
(10). Nishida S.Blumea. 2008. 53:345–383.
(11). Salleh W. M. N. H. W.., Ahmad F.., Yen K. H.., Zulkifli R. M.Pharm. Biol. 2016. 54:322–330.
(12). Tulake A.., Jiang Y.., Tu P. F. J.Chinese Pharm. Sci. 2012. 21:360–364.
(13). Russell G. B.., Fenemore P. G.Phytochemistry. 1973. 12:1799–1803. . 1803.
(14). Lee H. J.., Seo S. M.., Lee O. K.., Jo H. J.., Kang H. Y.., Choi D. H.., Paik K. H.., Khan M.Helv. Chim. Acta. 2008. 91:2361–2366.
(15). Yang P. S.., Cheng M. J.., Peng C. F.., Chen J. J.., Chen I. S. J.Nat. Prod. 2009. 72:53–58.
(16). Ahmed Y.., Sohrab M. H.., Al-Reza S. M.., Tareq F. S.., Hasan C. M.., Sattar M. A.Food Chem. Toxicol. 2010. 48:549–552.
(17). Chen J. J.., Chou E. T.., Duh C. Y.., Yang S. Z.., Chen I. S.Planta Med. 2006. 72:351–357.
(18). Ellman G. L.., Courtney K. D.., Andres V. Jr.., Feather-Stone R. M.Biochem. Pharmacol. 1961. 7:88–95.
(19). Orhan I.., Aslan S.., Kartal M.., Sener B.., Hüsnü Can Ba er K.Food Chem. 2008. 108:663–668.
(20). Bertanha C. S.., Braguine C. G.., Moraes A. C. G.., Gimenez V. M. M.., Groppo M.., Silva M. L. A.., Cunha W. R.., Januário A. H.., Pauletti P. M.Nat. Prod. Res. 2012. 26:2323–2329.
(21). Huang Y. T.., Chang H. S.., Wang G. J.., Cheng M. J.., Chen C. H.., Yang Y. J.., Chen I. S. J.Nat. Prod. 2011. 74:1875–1880.
(22). Mollataghi A.., Hadi A. H.., Awang K.., Mohamad J.., Litaudon M.., Mukhtar M. R.Molecules. 2011. 16:6582–6590.
(23). Thuong P. T.., Hung T. M.., Khoi N. M.., Nhung H. T.., Chinh N. T.., Quy N. T.., Jang T. S.., Na M. K.Arch. Pharm. Res. 2014. 37:399–403.
(24). Pan J. Y.., Zhang S.., Wu J.., Li Q. X.., Xiao Z. H.Helv. Chim. Acta. 2010. 93:951–957.
(25). Kim K. H.., Moon E.., Ha S. K.., Suh W. S.., Kim H. K.., Kim S. Y.., Choi S. U.., Lee K. R.Chem. Pharm. Bull. 2014. 62:1136–1140.
(26). Chen T. H.., Huang Y. H.., Lin J. J.., Liau B. C.., Wang S. Y.., Wu Y. C.., Jong T. T.Planta Med. 2010. 76:613–619.
(27). Barbosa-Filho J. M.., Vargas M. R. W.., Silva I. G.., Franca I. S.., Morais L. C. S. L.., Cunha E. V. L.., Silva M. S.., Souza M. F. V.., Chaves M. C. O.., Almeida R. N.., Agra M. F.Ann. Braz. Acad. Sci. 1999. 71:231–238.
(28). Ramassamy C.Eur. J. Pharmacol. 2006. 545:51–64.
(30). Kwak J. H.., Jeong C. H.., Kim J. H.., Choi G. N.., Shin Y. H.., Lee S. C.., Cho S. H.., Choi S. G.., Heo H. J.Korean J. Food Sci. Technol. 2009. 41:435–440.
(31). Roman G.Eur. J. Med. Chem. 2015. 89:743–816.
(32). de Gaetano G.., Donati M. B.., Cerletti C.Trends Pharmacol. Sci. 2003. 24:245–252.
(33). McGeer P. L.., McGeer E. G.Muscle Nerve. 2002. 26:459–470.
(34). Ling S. K.., Shaari K.., Ali R. M.., Ali N. A. M. J.Trop. Forest Prod. 2000. 4:192–198.
Go to : 
Table 1.
Anticholinesterase and anti-inflammatory activities of lignans from B. pulverulenta




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download