Abstract
The microbial transformation of anthranilic acid (1) by the marine-mudflat-derived fungus Thielavia sp. produced an antibacterial polycyclic quinazoline alkaloid, thielaviazoline (2). The stereostructure of the metabolite was assigned based on detailed spectroscopic data analyses including comparison of the NMR (1H and13C) data with those of reported compound (2). Compound 2 displayed in vitro antimicrobial activity against methicillin-resistant and multidrug-resistant Staphylococcus aureus (MRSA and MDRSA), with minimum inhibitory concentrations (MICs) of 6.25 and 12.5 µ g/mL, respectively. Compound 2 also showed potent radical-scavenging activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) with an IC50 of 11 µM, which was more active than the positive control, L-ascorbic acid (IC50, 20.0 µM).
Go to : 
REFERENCES
(2). El Sayed K. A., Hamann M. T., Waddling C. A., Jensen C., Lee S. K., Dunstan C. A., Pezzuto J. M. J.Org. Chem. 1998; 63:7449–7455.
(3). Li X., Lee S. M., Choi H. D., Kang J. S., Son B. W.Chem. Pharm. Bull. 2003; 51:1458–1459.
(4). Li X., Kim S. -K., Jung J. H., Kang J. S., Choi H. D., Son B. W.Bull. Korean Chem. Soc. 2005; 26:1889–1890.
(5). Li X., Kim Y. H., Jung J. H., Kang J. S., Kim D. -K., Choi H. D., Son B. W.Enz. Microbial Technol. 2007; 40:1188–1192.
(6). Leutou A. S., Yang G., Nenkep V. N., Siwe X. N., Feng Z., Khong T. T., Choi H. D., Kang J. S., Son B. W. J.Microbiol. Biotechnol. 2009; 19:1150–1152.
(7). Feng Z., Nenkep V., Yun K., Zhang D., Choi H. D., Kang J. S., Son B. W. J.Microbiol. Biotechnol. 2010; 20:985–987.
(8). Yun K., Kondempudi C. M., Choi H. D., Kang J. S., Son B. W.Chem. Pharm. Bull. 2011; 59:499–501.
(9). Leutou A. S., Yun K., Son B. W.Bull. Korean Chem. Soc. 2014; 35:2870–2872.
(10). Yun K., Kondempudi C. M., Leutou A. S., Son B. W.Bull. Korean Chem. Soc. 2015; 36:2391–2393.
(11). The Chemical Daily Co. 10, 889 of Chemical Products. The Chemical Daily Co., Ltd.;Japan: 1989. p. 499–500.
(12). Li Y., Li X., Son B. W.Nat. Prod. Sci. 2005; 11:136–138.
(14). Manske R. H. F., Holmes H. L.The Alkaloids – Chemistry and Pharmacology. Vol. III; Academic Press: USA. 1953; 101–111.
(15). Xiao X., Fanwick P. E., Cushman M.Synth. Commun. 2004; 34:3901–3907.
(16). Younes E. A., Hussein A. Q., May M. A., Fronczek F. R.ARKIVOC. 2011; 2:323–330.
Go to : 
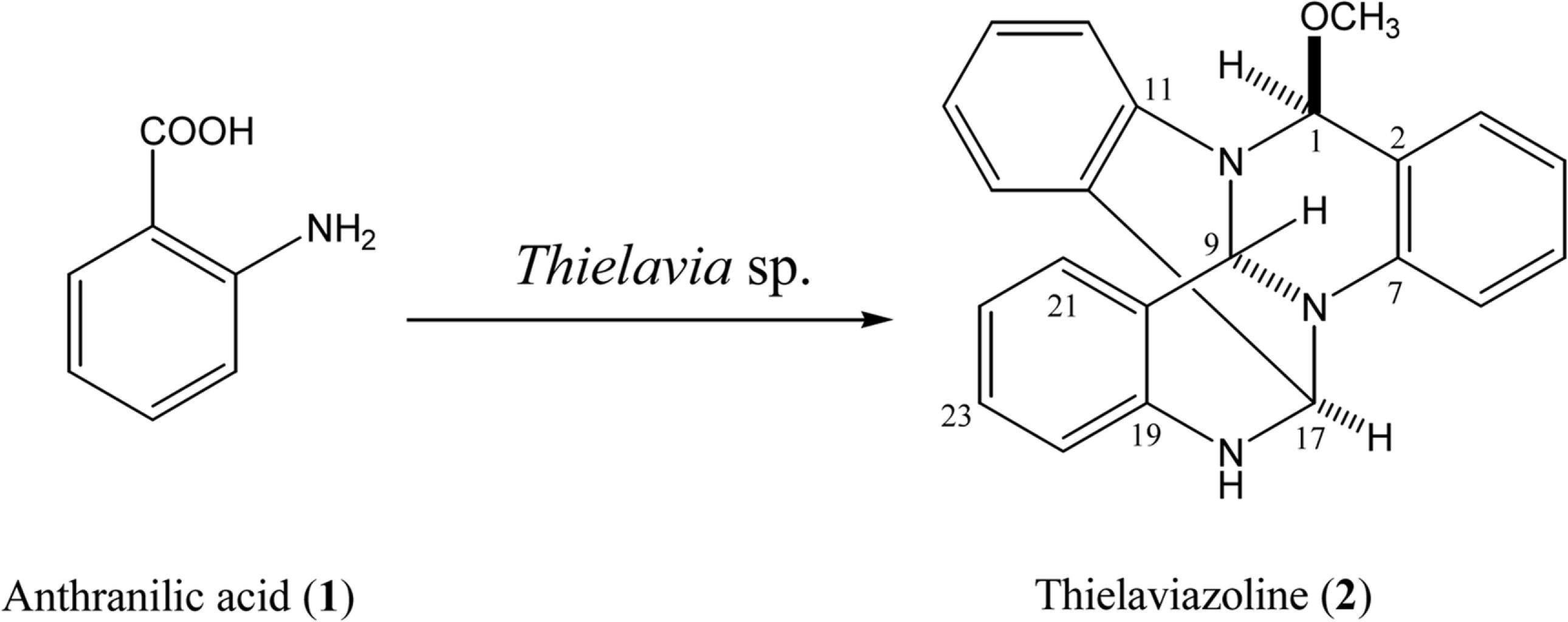 | Fig. 1.Microbial synthesis of thielaviazoline (2) from anthranilicacid (1) by the marine-mudflat-derived fungus Thielavia sp. |
Table 1.
NMR spectra data for thielaviazoline (2)a
| thielaviazoline(2) | |||
|---|---|---|---|
| Position | δH (mult, J) | δC (mult) | HMBC (H to C) |
| 1 | 5.09 (s) | 693.1 (d) | C-2, -3, -7, -9, -11, 1-OMe |
| 2 | 126.7 (s) | ||
| 3 | 7.23 (m)b | 129.7 (d) | C-1, -2, -5, -7 |
| 4 | 7.02 (m)c | 123.7 (d)α | C-2, -6 |
| 5 | 7.21 (m)b | 123.5 (d) | C-3, -7 |
| 6 | 7.14 (m)d | 128.0 (d) | C-2, -4 |
| 7 | 144.9 (s) | ||
| 8-N | |||
| 9 | 5.23 (s) | 662.7 (d) | C-1, -7, -11, -17, -19, -20, -21 |
| 10-N | |||
| 11 | 142.3 (s) | ||
| 12 | 7.03 (m)c | 128.5 (d)β | C-14, -16 |
| 13 | 7.09 (m) | 128.4 (d)β | |
| 14 | 7.02 (m)c | 123.8 (d)α | |
| 15 | 7.12 (m)d | 128.8 (d)γ | C-11, -13, -17 |
| 16 | 130.0 (s) | ||
| 17 | 5.36 (d,4.3) | 668.3 (d) | C-7, -9, -11, -19 |
| 18-NH | 7.05 (m)c | C-16, -17, -19, -20, -24 | |
| 19 | 142.0 (s) | ||
| 2021 | 7.25 (m)b | 120.6 (s)128.9 (d)γ | C-9, -19, -23 |
| 22 | 6.69 (dd,7.5, 7.3) | 121.2 (d) | |
| 23 | 6.94 (dd,7.5, 7.3) | 123.9 (d) | |
| 24 | 6.64 (d,8.0) | 115.6 (d) | C-19, -20, -22 |
| 1-OMe | 3.82 (s) | 654.9 (q) | C-1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download