Abstract
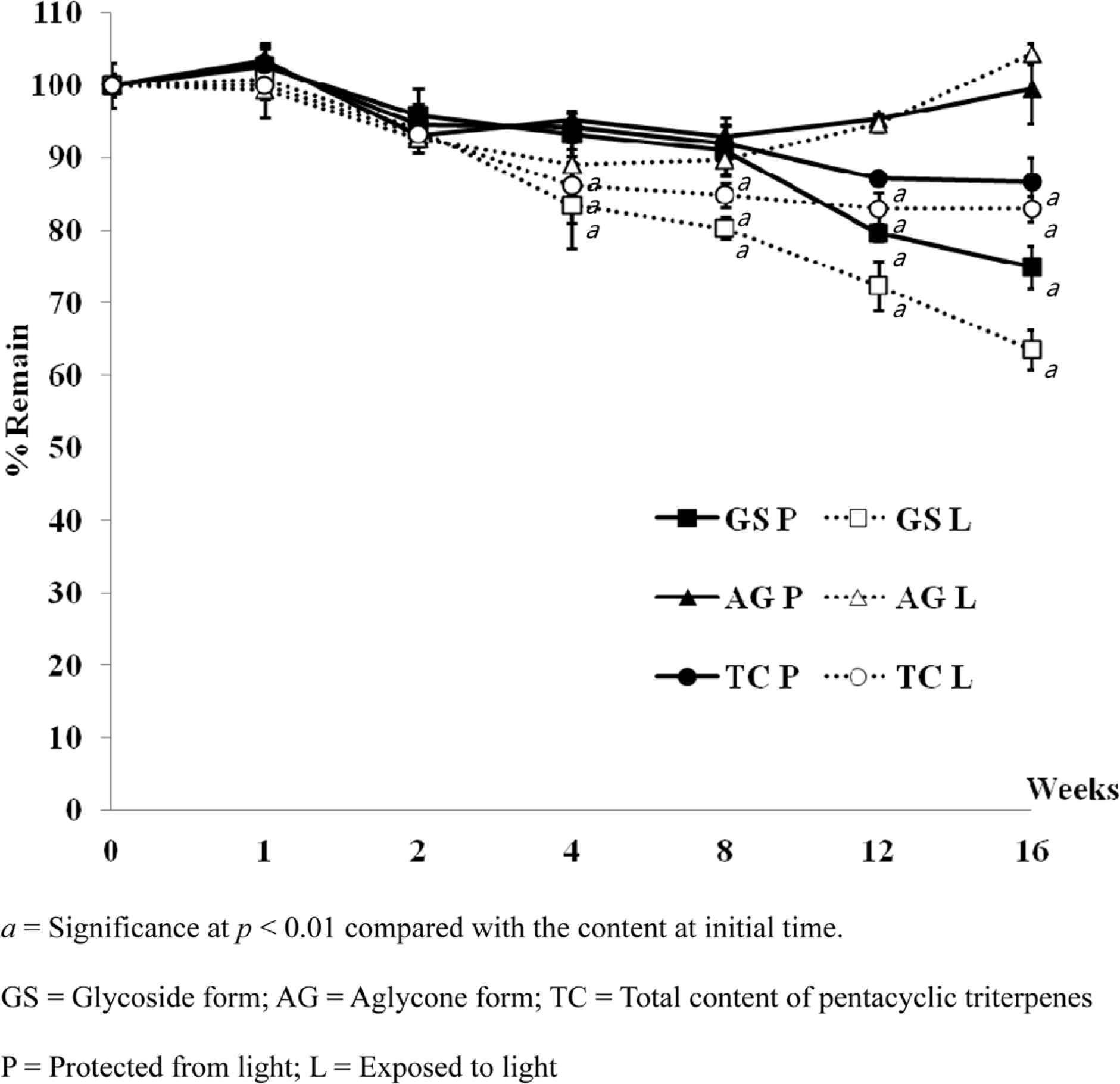
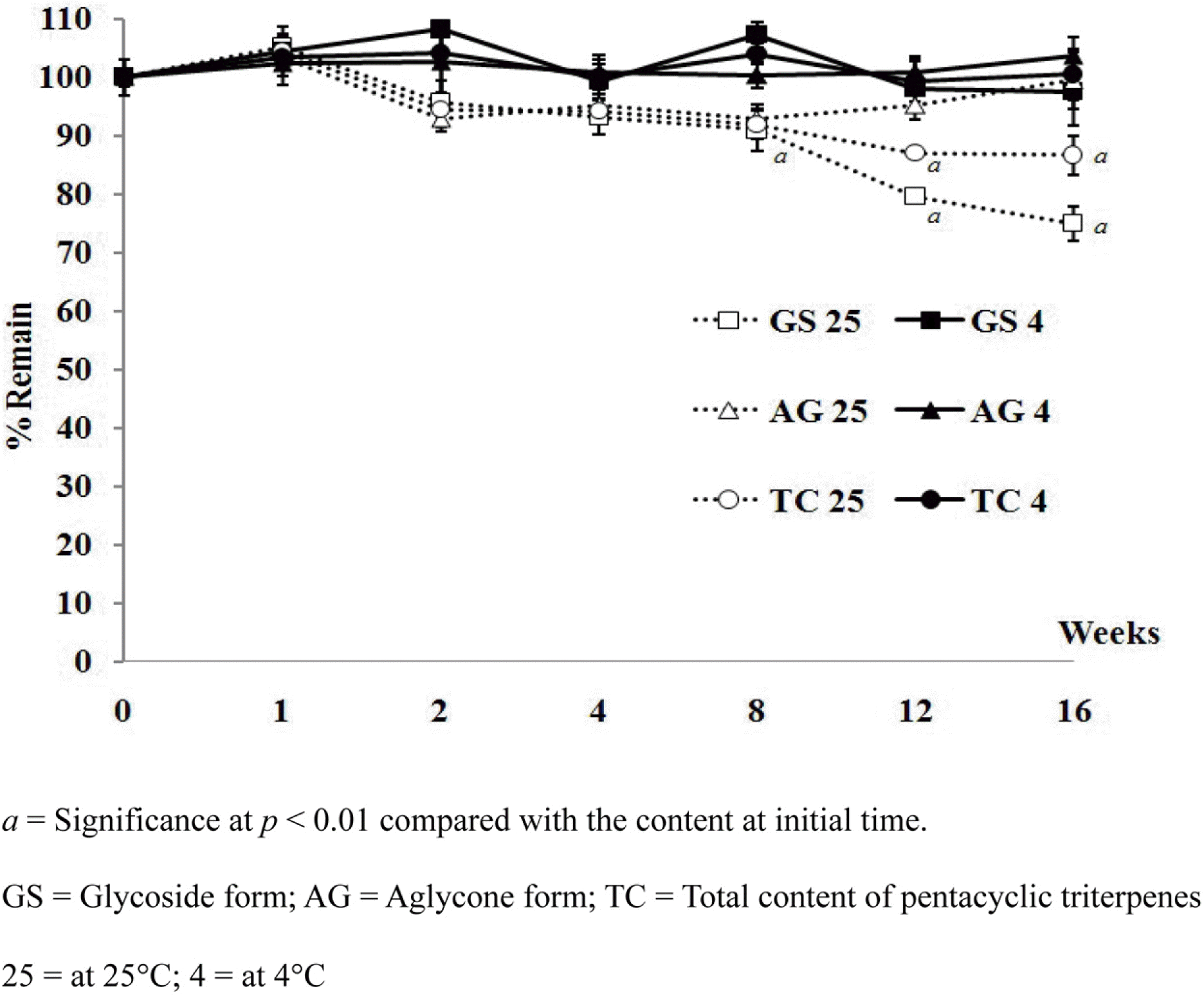
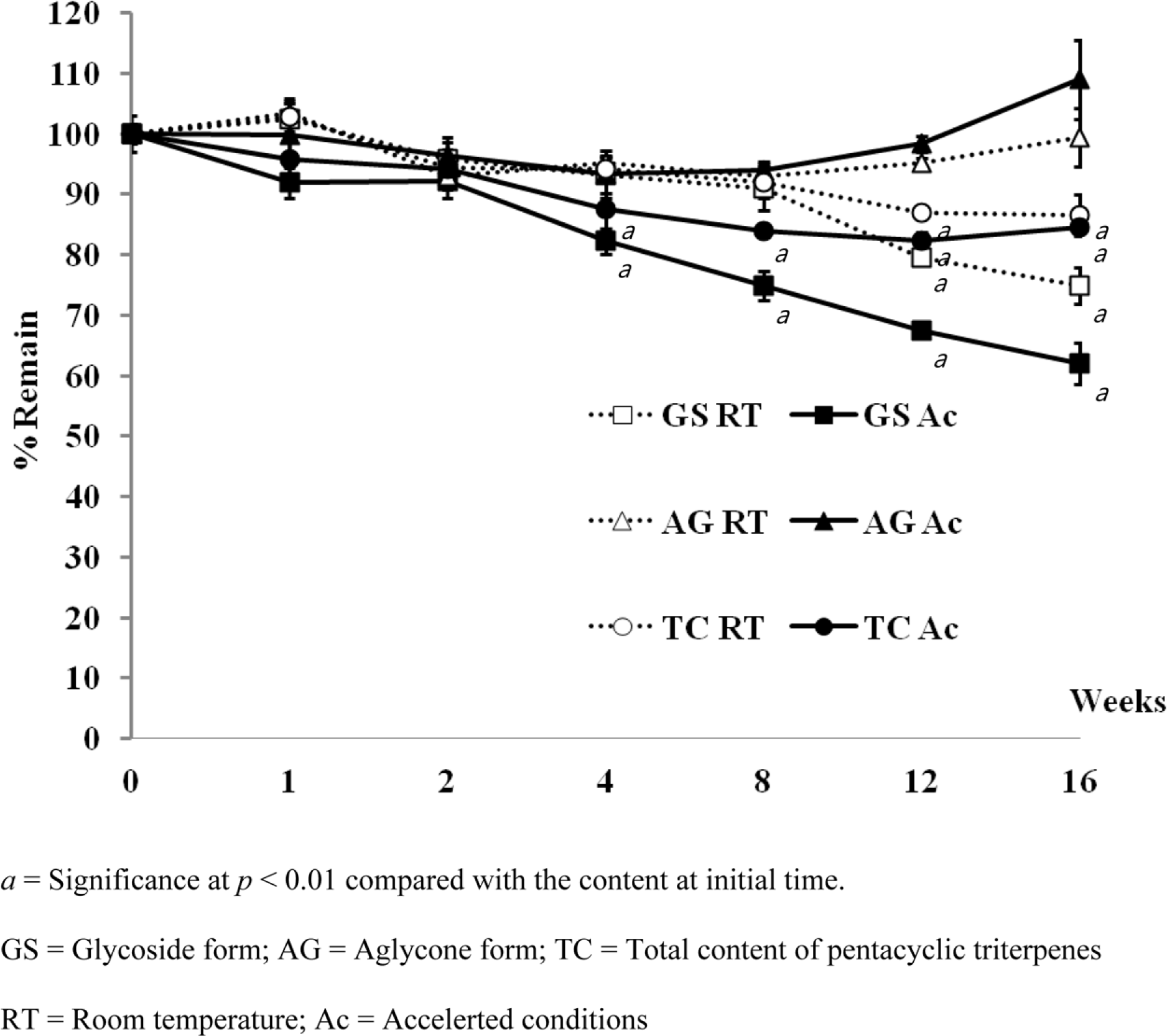
Pentacyclic triterpenes, mainly, asiatic acid, madecassic acid, asiaticoside, and madecassoside are the active constituents of Centella asiatica. A pentacyclic triterpene enriched C. asiatica extract (PRE) was prepared and standardized to contain a total pentacyclic triterpenes not less than 65% w/w. This work was focused on determination of antiinflammatory, antioxidant, and tyrosinase inhibitory activities of PRE and its stability. The PRE exhibited a satisfactory nitric oxide inhibitory effect, with an IC50 value of 64.6 µg/mL. In addition, the PRE inhibited tyrosinase enzyme activity with an IC50 value of 104.8 µg/mL. In contrast, the PRE possessed only weak antioxidant activity. The PRE was stable over a period of four months when stored as a dried powder but only in a well-closed container protected from light at 4oC. An aqueous alcoholic solution of the PRE was stable at pH values of 5.8 and 7.0, but was not stable at a pH of 8.2. Preparations of the PRE in an aqueous solution should be performed in acidic or neutral conditions.
Go to : 
REFERENCES
(1). Bonte F., Dumas M., Chaudagne C., Meybeck A.Planta Med. 1994; 60:133–135.
(2). Maquart F. X., Bellon G., Gillery P., Wegrowski Y., Borel J. P.Connect. Tissue Res. 1990; 24:107–120.
(3). Yun K. J., Kim J. Y., Kim J. B., Lee K. W., Jeong S. Y., Park H. J., Jung H. J., Cho Y. W., Yun K., Lee K. T.Int. Immunopharmacol. 2008; 8:431–441.
(4). Won J. H., Shin J. S., Park H. J., Jung H. J., Koh D. J., Jo B. G., Lee J. Y., Yun K., Lee K. T.Planta Med. 2010; 76:251–257.
(5). Cheng C. L., Guo J. S., Luk J., Koo M. W. L.Life Sci. 2004; 74:2237–2249.
(6). Puttarak P., Panichayupakaranant P.Nat. Prod. Res. 2013; 27:684–686.
(7). Park B. C., Bosire K. O., Lee E. S., Lee Y. S., Kim J. A.Cancer Lett. 2005; 218:81–90.
(8). Kaewchoothong A., Tewtrakul S., Panichayupakaranant P.Phytother. Res. 2012; 26:1789–1792.
(9). Panichayupakaranant P., Itsuriya A., Sirikatitham A.Pharm. Biol. 2010; 48:201–205.
(10). Burits M., Bucar F.Phytother. Res. 2000; 14:323–328.
(11). Rangkadilok N., Sitthimonchai S., Worasuttayangkurn L., Mahidol C., Ruchirawat M., Satayavivad J.Food Chem. Toxicol. 2007; 45:328–336.
(12). Puttarak P., Charoonratana T., Panichayupakaranant P.Phytomedicine. 2010; 17:323–327.
(13). Pittella F., Dutra R. C., Junior D. D., Lopes M. T., Barbosa N. R.Int. J. Mol. Sci. 2009; 10:3713–3721.
(14). Ariffin F., Chew S. H., Bhupinder K., Karim A. A., Huda N. J.Sci. Food Agric. 2011; 91:2731–2739.
(15). Subban R., Veerakumar A., Manimaran R., Hashim K. M., Balachandran I. J.Nat. Med. 2008; 62:369–373.
(16). Kubo I., Kinst-Hori I., Chaudhuri S.K., Kubo Y, Sánchez Y, Ogura T.Bioorg. Med. Chem. 2000; 8:1749–1755.
Go to : 
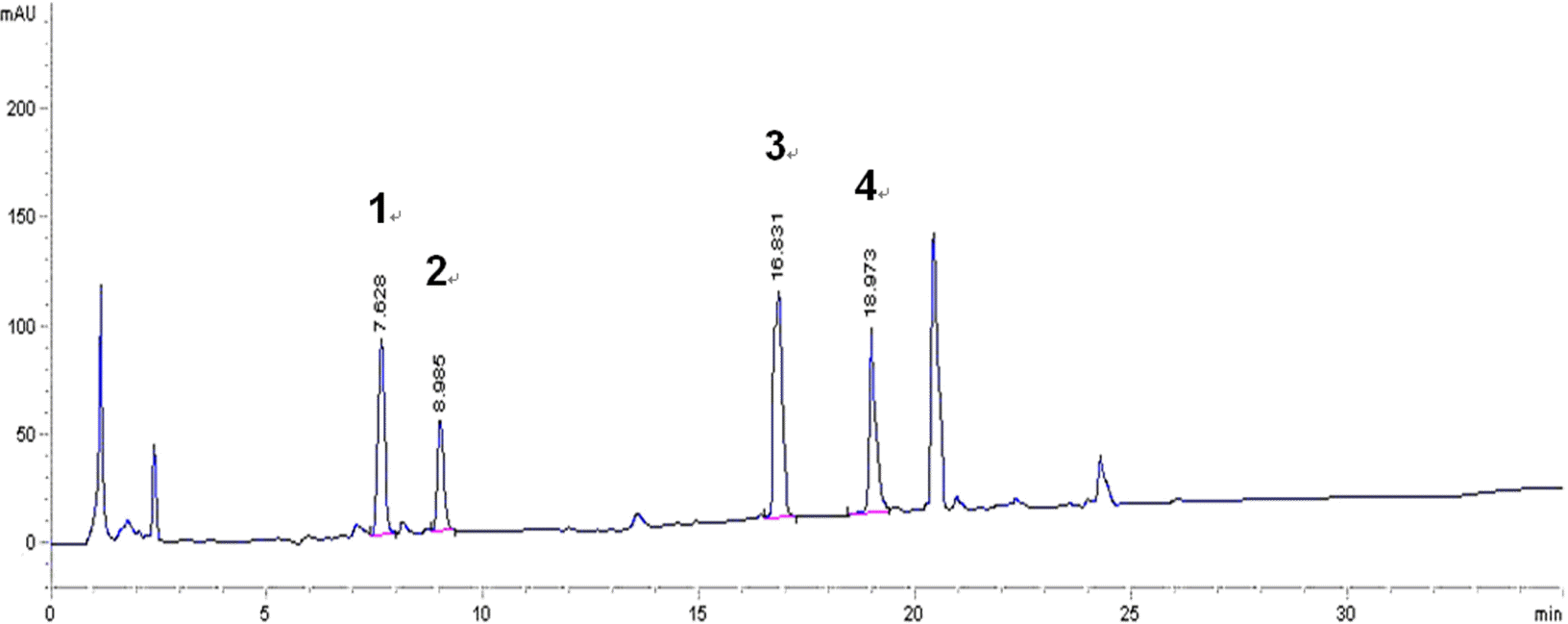 | Fig. 1.HPLC chromatogram of the standardized PRE. Madecassoside (1); asiaticoside (2); madecassic acid (3); asiatic acid (4). |
Table 1.
Biological activities of the PRE and four pure pentacyclic triterpenes from C. asiatica
| Samples | IC50 µg/mL (µM) | |||
|---|---|---|---|---|
| NO inhibition | DPPH | Lipid peroxidation | Tyrosinase inhibition | |
| PRE | 64.6 | 348.3 | n.a.# | 104.8 |
| Asiatic acid | 12.4 (25.4) | n.a.∗ | n.a.# | n.a.# |
| Madecassic acid | n.a.+ | n.a.∗ | n.a.# | n.a.# |
| Asiaticoside | n.a.+ | n.a.∗ | n.a.# | n.a.# |
| madecassoside | n.a.+ | n.a.∗ | n.a.# | n.a.# |
| Aspirin | 12.9 (71.7) | − | − | − |
| CAPE | 1.6 (5.6) | − | − | − |
| BHT | − | 60.9 (276.3) | − | − |
| Qurcetin | − | 1.7 (5.6) | 1.7 (5.6) | − |
| Kojic acid | − | − | − | 5.0 (35.2) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download