Abstract
Anti-melanogenic effects of amaranth (AT), one of the key source of squalene, were investigated in melanocytes. Amaranth seed powder was extracted with water and melan-a cells were treated with various concentrations of AT. By using HPLC, content of myo-inositol, one of potential active components, was measured in the crude extract of AT.AT reduced the melanin content in melan-a melanocytes and down-regulated melanogenic enzyme activity such as tyrosinase, TRP-1 and TRP-2. By regulating melanogenic enzyme activity, AT may be a potential natural source for whitening agent. Myo-inositol was detected in AT by HPLC and may be one of the active compounds from AT involved in the regulation of anti-melanogenesis. In this study, we demonstrated that AT has anti-melanogenesis properties. This new function of amaranth may be useful in the development of new skin-whitening products and its value as food.
Go to : 
REFERENCES
(1). Spritz R. A, Hearing V. J. Jr.Adv Hum Genet. 1994; 22:1–45.
(2). Kadekaro A. L., Chen J., Yang J., Chen S., Jameson J., Swope V. B., Cheng T., Kadakia M., Abdel-Malek Z.Mol. Cancer Res. 2012; 10:778–786.
(3). Chou W. C., Takeo M., Rabbani P., Hu H., Lee W., Chung, Y. R.;Carucci J., Overbeek P., Ito M.Nat. Med. 2013; 19:924–929.
(4). Wasmeier C., Hume A. N., Bolasco G., Seabra M. C. J.Cell Sci. 2008; 121:3995–3999.
(5). Kim H. J., Lee J. H., Shin M. K., Hyun Leem K., Kim Y. J., Lee M. H. J.Cosmet. Sci. 2013; 64:89–98.
(6). Lee T. H., Seo J. O., Baek S. H., Kim S. Y.Biomol Ther (Seoul). 2014; 22:35–40.
(7). Bertolotto C., Abbe P., Hemesath T. J., Bille K., Fisher D. E., Ortonne J. P., Ballotti R. J.Cell Biol. 1998; 142:827–835.
(8). Bentley N. J., Eisen T., Goding C. R.Mol. Cell Biol. 1994; 14:7996–8006.
(9). Chang T. S.Int. J. Mol. Sci. 2009; 10:2440–2475.
(10). Lucas A. M., Thody A. J., Shuster S.Peptides. 1987; 8:955–960. 1995.
(11). Orlow S. J. J.Invest. Dermatol. 1995; 105:3–7.
(12). Jimenez-Cervantes C., Garcia-Borron J. C., Valverde P., Solano F., Lozano J. A.Eur. J. Biochem. 1993; 217:549–556.
(13). Mars U., Larsson B. S.Pigment. Cell Res. 1995; 8:194–201.
(14). Kamakshi R. J.Cosmet. Sci. 2012; 63:43–54.
(15). Kobayashi T., Urabe K., Winder A., Jiménez-Cervantes C., Imokawa G., Brewington T., Solano F., García-Borrón J. C., Hearing V. J.EMBO. J. 1994; 13:5818–5825.
(16). Moon E., Kim A. J., Kim S. Y.Biomol. Ther (Seoul). 2012; 20:340–345.
(17). Cordell G. A., Colvard M. D. J.Nat. Prod. 2012; 75:514–525.
(18). Jung S., Lee J. H., Lee Y. C., Moon H. I.Immunopharmacol. Immunotoxicol. 2012; 34:210–212.
(19). Perales-Sánchez J. X., Reyes-Moreno C., Gómez-Favela M. A., Milán-Carrillo J., Cuevas-Rodríguez E. O., Valdez-Ortiz A., Gutiérrez-Dorado R.Plant Foods Hum. Nutr. 2014; 69:196–202.
(20). López V. R., Razzeto G. S., Giménez M. S., Escudero N. L.Plant Foods Hum. Nutr. 2011; 66:157–162.
(21). Kraujalis P., Venskutonis P. R., Kraujalien V., Pukalskas A.Plant Foods Hum. Nutr. 2013; 68:322–328.
(22). Pedersen H. A., Steffensen S. K., Christophersen C., Mortensen A. G., Jørgensen L. N., Niveyro S., de Troiani R. M., Rodríguez-Enríquez R. J., Barba-de la Rosa A. P., Fomsgaard I. S. J.Agric. Food Chem. 2010; 58:6306–6311.
(23). Tikekar R. V., Ludescher R. D., Karwe M. V. J.Agric. Food Chem. 2008; 56:10675–10678.
(24). Rodas B., Bressani R.Arch. Latinoam. Nutr. 2009; 59:82–87.
(25). Jiménez-Cervantes C., Martínez-Esparza M., Pérez C., Daum N., Solano F., García-Borrón J. C. J.Cell Sci. 2001; 114:2335–2344.
(26). López-Mejía O. A., López-Malo A., Palou E.Arch. Latinoam. Nutr. 2014; 64:50–58.
(27). Medoua G. N., Oldewage-Theron W. H. J.Food Sci. Technol. 2014; 51:736–742.
(28). Hosoi J., Abe E., Suda T., Kuroki T.Cancer Res. 1985; 45:1474–1478.
(29). Kunyanga C. N., Imungi J. K., Okoth M., Momanyi C., Biesalski H. K., Vadivel V. J.Food Sci. 2011; 76:C560–567.
(30). Huang H. C., Hsieh W. Y., Niu Y. L., Chang T. M.Int. J. Mol. Sci. 2014; 15:16665–16679.
(31). Shi Y., Luo L. F., Liu X. M., Zhou Q., Xu S. Z., Lei T. C.PLoS One. 2014; 9:e93589.
(32). Caselato-Sousa V. M., Amaya-Farfán J. J.Food Sci. 2012; 77:R93–104.
(33). Jun H. J., Roh M., Kim H. W., Houng S. J., Cho B., Yun E. J., Hossain M. A., Lee H., Kim K. H., Lee S. J.Food Sci.Biotechnol. 2011; 20:1051–1059.
(35). Kim D. S., Kim S. Y., Park S. H., Choi Y. G., Kwon S. B., Kim M. K., Na J. I., Youn S. W., Park K. C.Biol. Pharm. Bull. 2005; 28:2216–2219.
(36). Sun B. B., Qi L., Mu X. Y., Qiao J., Wang M. L.Talanta. 2013; 116:1121–1125.
Go to : 
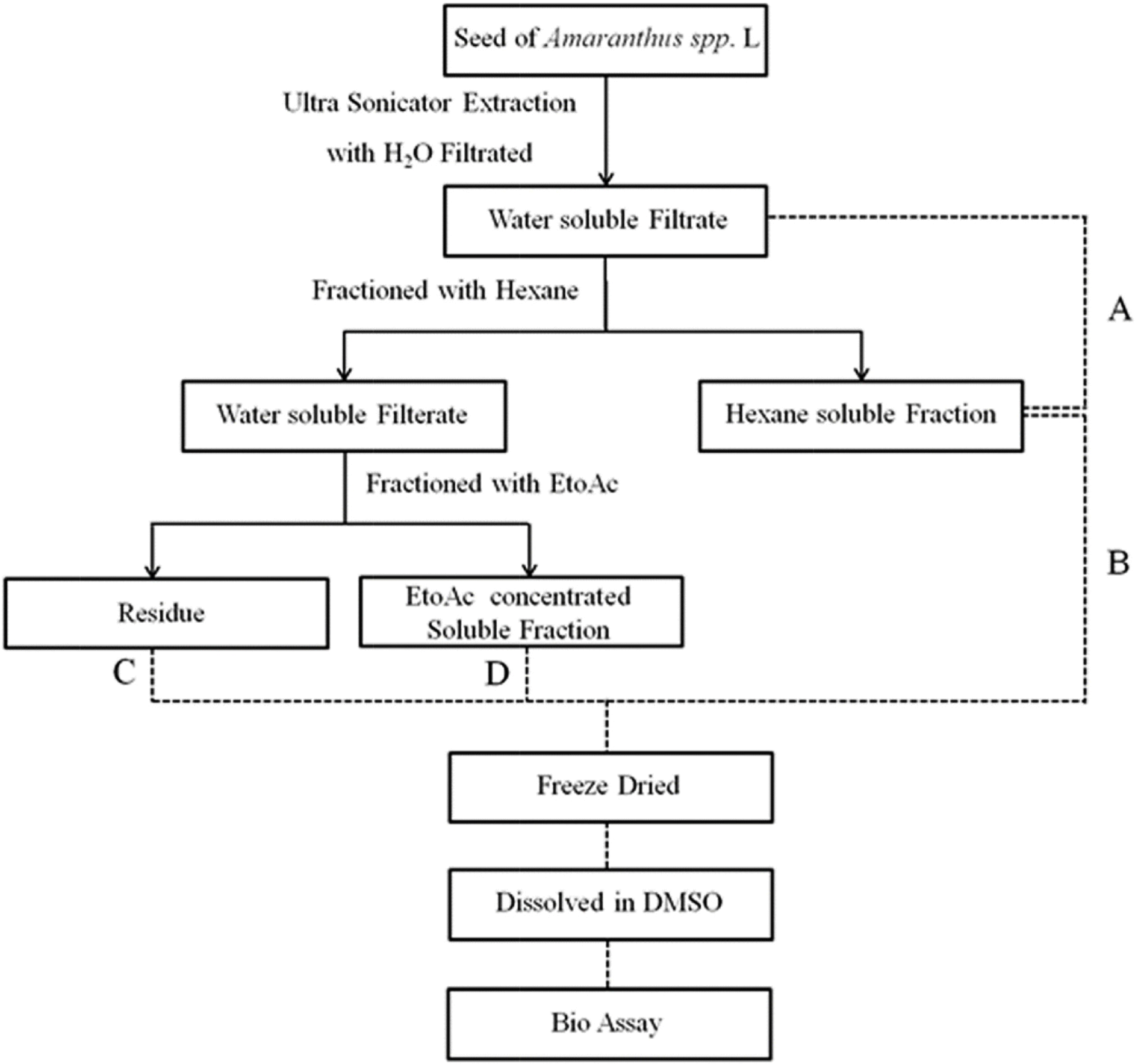 | Fig. 1.Scheme of sample preparation. Simply present the steps of extraction of AT. A: Amaranthus spp. L Total extract, B: Hexane soluble fraction, C: Residue, D: EtoAc soluble fraction. |
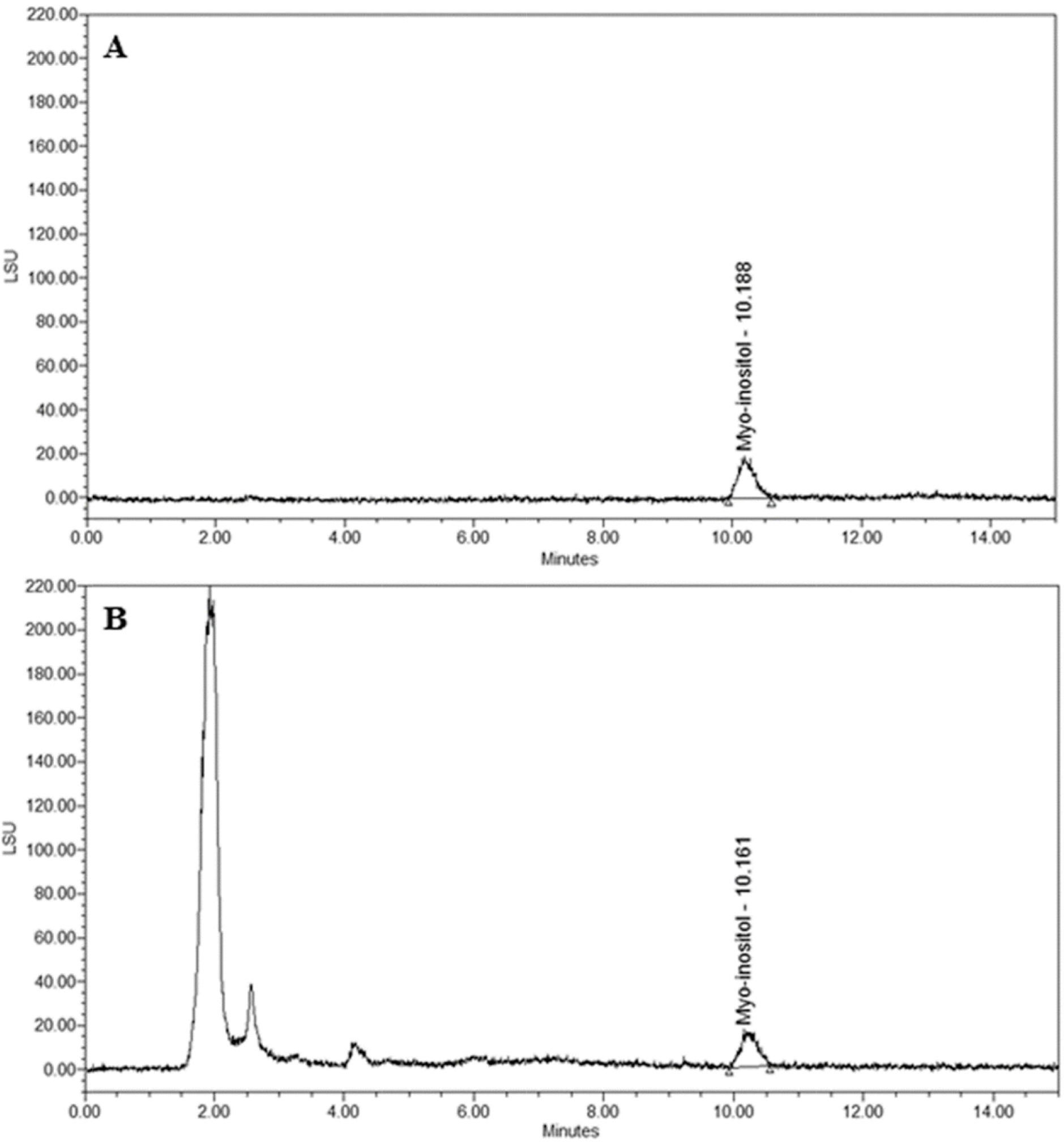 | Fig. 2.HPLC chromatogram for the determination of myo-inositol in Amaranth ushypocondriacus (A.D). A. Chromatogram of myo-inositol B. Chromatogram of AD. |
 | Fig. 3.Effects of Amaranth seed extract (AT) on melanin content and cell viability in melan-a cells. Melanin contents (A) and cell viability (B) were measured. CTL indicates normal conditions and Arbu indicate the use of arbutin as a positive control (μM). All sample (μ g/mL) were treated for 72 h. Data are expressed as mean ± S.D. of three experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 compared with control. |
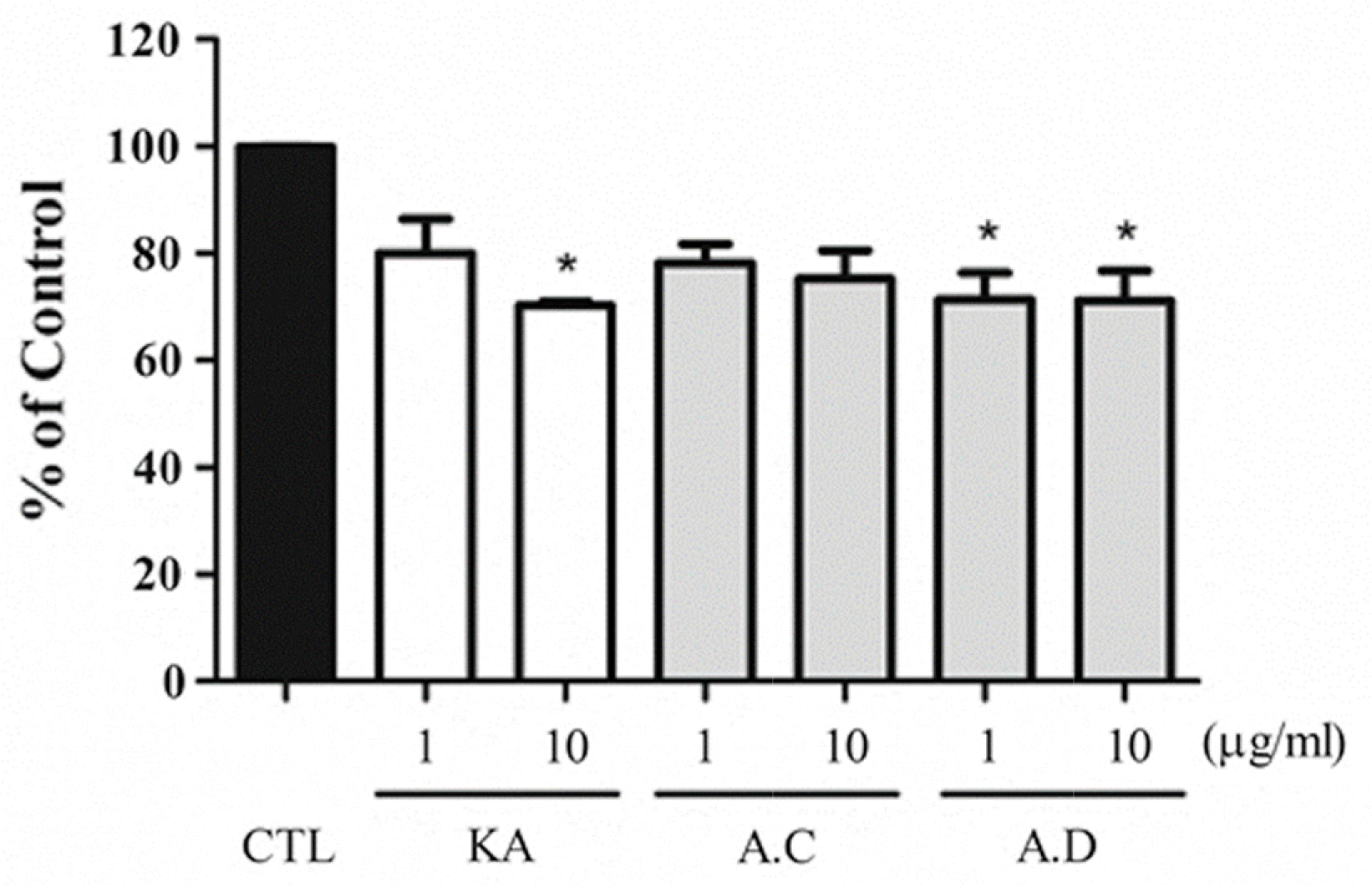 | Fig. 4.Effects of AT on tyrosinase activity in melan-a cells. Melan-a cell originated tyrosinase activity was measured. CTL indicates normal conditions and KA indicate the use of kojic acid as a positive control (μM). Each samples were treated with μg/ mL. The results are expressed as mean ± S.D. of three experiments (∗p < 0.05). |
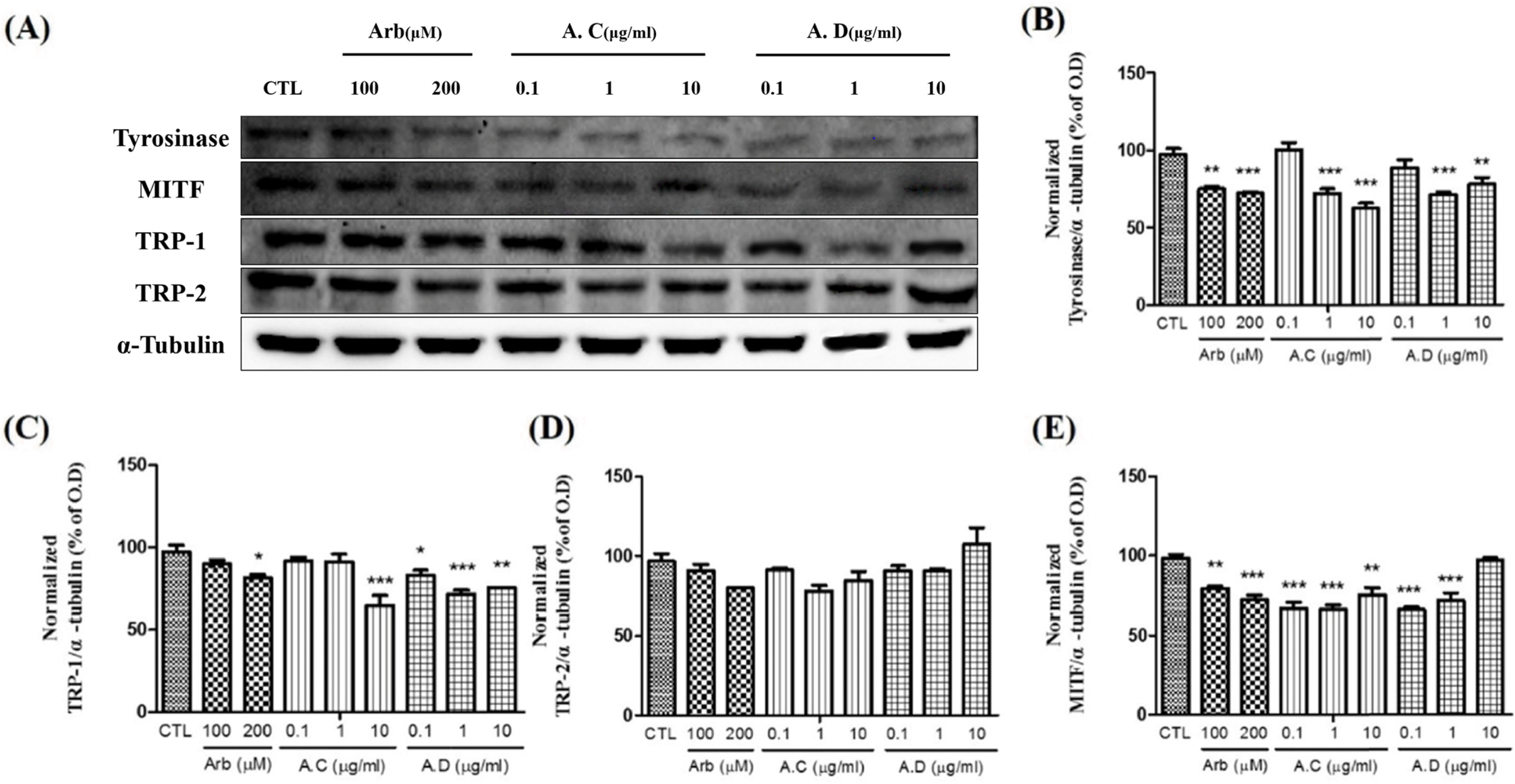 | Fig. 5.Effects of Amaranth ushybridus (A.C) and Amaranth ushypocondriacus (A.D) on the expression of melanogenic enzymes in melan-a cells. To confirm the expression of melanogenic enzymes, melan-a cells were treated with 0.1, 1 and 10 μg/mL of A.C and A.D. Arbutin was used as a positive control (μ M). (A) Western blot analysis. Densitometric analysis of Tyrosinase (B), TRP-1 (C), TRP-2 (D) and MITF (F). Each band was normalized to that of α-tubulin. |
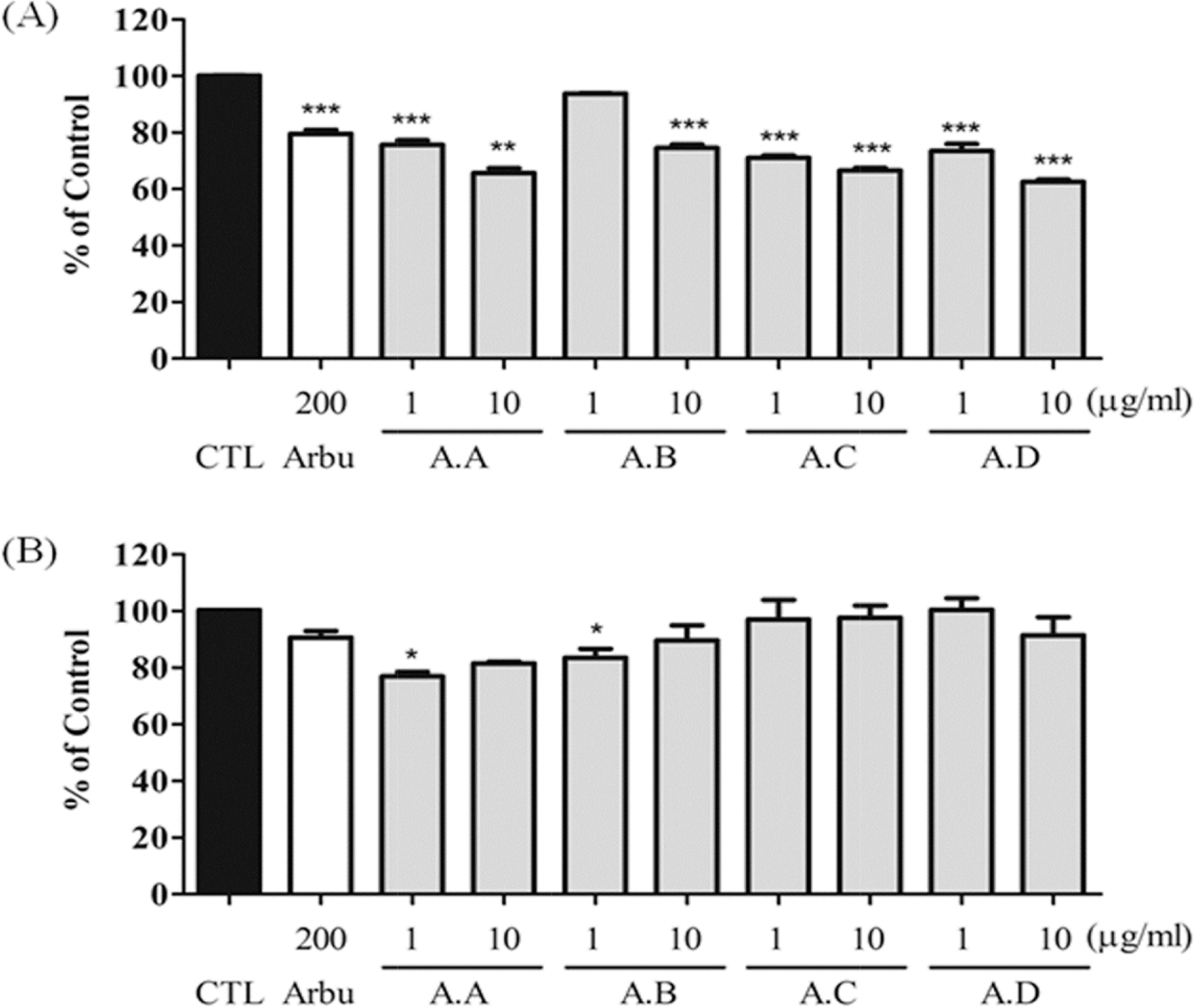
 | Fig. 6.Effects of each fraction of amaranth seed extract (AT) on melanin content and cell viability in melan-a cells Melanin contents (A) and cell viability (B) were measured. CTL indicates normal conditions and Arbu indicate the use of arbutin as a positive control (μM). All sample (μg/mL) were treated for 72 h. Data are expressed as mean ± S.D. of three experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 compared with control. |
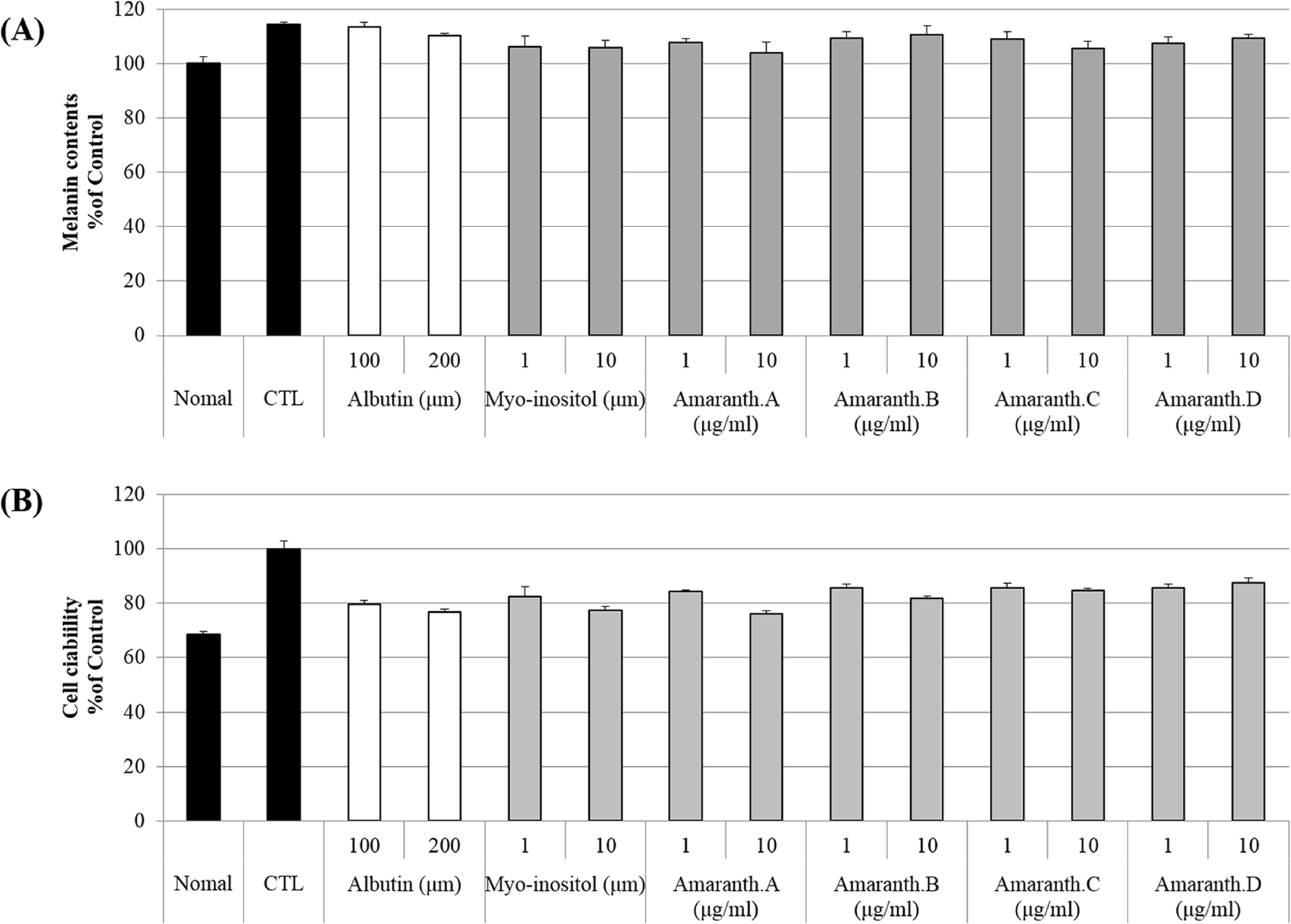 | Supplement 1.Effects of Amaranth species on melanin content and cell viability in mouse melanoma B16F10 cells. Cell viability (A) and melanin contents (B) were measured. B16F10 cells were pre-stimulated by α-MSH (100 ng/ml). After treatment of α-MSH for 30 min, all sample were treated for 72 h. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download