Abstract
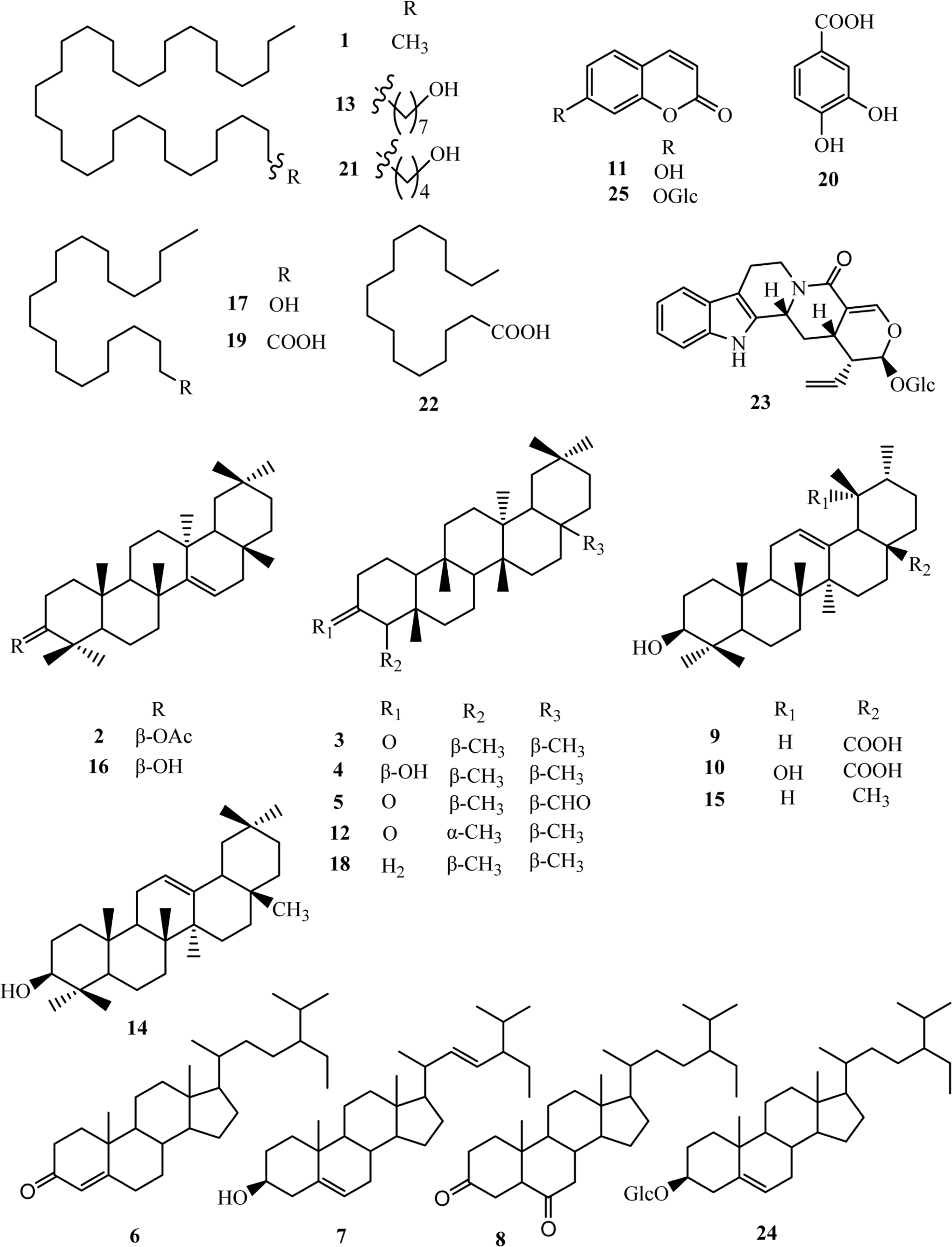
Phytochemical investigation on the leaves of Pileostegia viburnoides Hook.f.et Thoms led to the isolation of twenty-five compounds, and their structures were identified as n-dotriacontane (1), taraxeryl acetate (2), friedelin (3), epifriedelinol (4), canophyllal (5), stigmast-4-en-3-one (6), stigmasterol (7), (24R)-5A-stigmastane-3,6-dione (8), ursolic acid (9), pomolic acid (10), umbelliferone (11), 4-epifriedelin (12), n-octatriacontanol (13),β-amyrin (14), α-amyrin (15), taraxerol (16), nonadecanol (17), friedelane (18), arachic acid (19), protocatechuic acid (20), n-pentatriacontanol (21), hexadecanoic acid (22), vincosamide (23), daucosterol (24), and skimming (25), respectively. To our best knowledge, compounds 1, 2, 12, 13, 17–19 and 21-23 were new within Saxifragaceae family. Compounds 15, 16, and 20 were produced from this genus for the first time. Compounds 4,14 and 25 were first obtained from species P. viburnoides and compounds 3, 5–11, and 24 were achieved from the leaves of P. viburnoides for the first time. Furthermore, the anti-neuroinflammatory activity of these isolates was evaluated.
Go to : 
REFERENCES
(1). Cai G. X., Zhou S., Tan G. B.Hunan Yaowuzhi: Hunan Science and Technology Press: Changsha. 2004; 3360–3361.
(3). Li X. J., Yuan Y., Li Z., Liu X. Q.Chinese Traditional and Herbal Drugs. 2014; 45:1052–1055.
(4). Yuan Y., Liu X. Q., Liu Z. Z., Zou Q. P., Liu H. Y., Dai L.Journal of Chinese Medicinal Materials. 2011; 34:1894–1897.
(5). Yang D. D., Liu X. Q., Liu Z. Z., Zou Q. P., Liu H. Y., Dai L.Northwest Pharmaceutical Journal. 2012; 27:189–192.
(6). Pan X. X., Guo J. S., Guo Z. Y., Li Z., Nie J.Lishizhen Medicine and Materia Medica Research. 2012; 23:1920–1921.
(7). Qin L. H., Zhong H. M., Chen X. Y., Wu Y. F., Li Z., Huang K.Chinese Journal of Gerontology. 2014; 34:429–431.
(8). Titheradge M. A.Methods Mol. Biol. 1998; 100:83–91.
(9). Chang C. W., Wu T. S., Hsieh Y. S., Kuo S. C., Chao P. D. J.Nat. Prod. 1999; 62:327–328.
(10). Zhang Q. J., Yang X. S., Zhu H. Y., Hao X. J.Journal of Guizhou University (Natural Sciences). 2006; 23:270–271.
(11). Matsunaga S., Tanaka R., Akagi M.Phytochemistry. 1988; 27:535–537.
(12). Leong Y. W., Harrison L. J.Phytochemistry. 1999; 50:849–857.
(13). Kundu J. K., Rouf A. S., Hossain M. N., Hasan C. M., Rashid M. A.Fitoterapia. 2000; 71:577–579.
(14). Patra A., Chaudhuri S. K.Indian J. Chem. 1989; 28B:376–380.
(15). Sun L. L., Zhong Y., Xia H. M., Zhou Q., Jia L. V.Chin. Herb. Med. 2013; 5:1–4.
(16). Chen I. S., Lin Y. C., Tsai I. L., Teng C. M., Ko F. N., Ishikawa T., Ishii H.Phytochemistry. 1995; 39:1091–1097.
(17). He Z., Zhao M., Zong Y. Y., Cai S. X., Che Z. T.Chinese Traditional and Herbal Drugs. 2010; 41:36–39.
(18). Tian M. Y., Peng L. J., Feng T. T., Chen L., Zhou Y.Journal of Mountain Agriculture and Biology. 2014; 33:089–091.
(19). Yue J. R., Feng D. Q., Xu Y. K.Nat. Prod. Res. 2015; 29:1228–1234.
(20). Xu W. Q., Gong X. J., Zhou X., Mei X. P., Chen B. B.Chinese Traditional and Herbal Drugs. 2010; 41:1056–1060.
(21). Ya Q. K., Lu W. J., Chen J. Y., Tan X.Journal of Chinese Medicinal Materials. 2010; 33:1254–1256.
(22). Chen Y., Wei H. C., Wei Z., Zheng X. R., Zhang L., Zhou Z.Lishizhen Medicine and Materia Medica Research. 2012; 23:1725–1726.
(23). Erdelmeier C. A., Wright A. D., Orjala J., Baumgartner B., Rali T., Sticher O.Planta Med. 1991; 57:149–152.
(24). He Y. Z., Eric K. O., Sivoko I. P., Wang L. N., Naji S. A.Chin. Herb. Med. 2014; 6:76–79.
(25). Petrul'ová-Poracká V., Rep ák M., Vilková M., Imrich J.Food c Chem. 2013; 141:54–59.
Go to : 
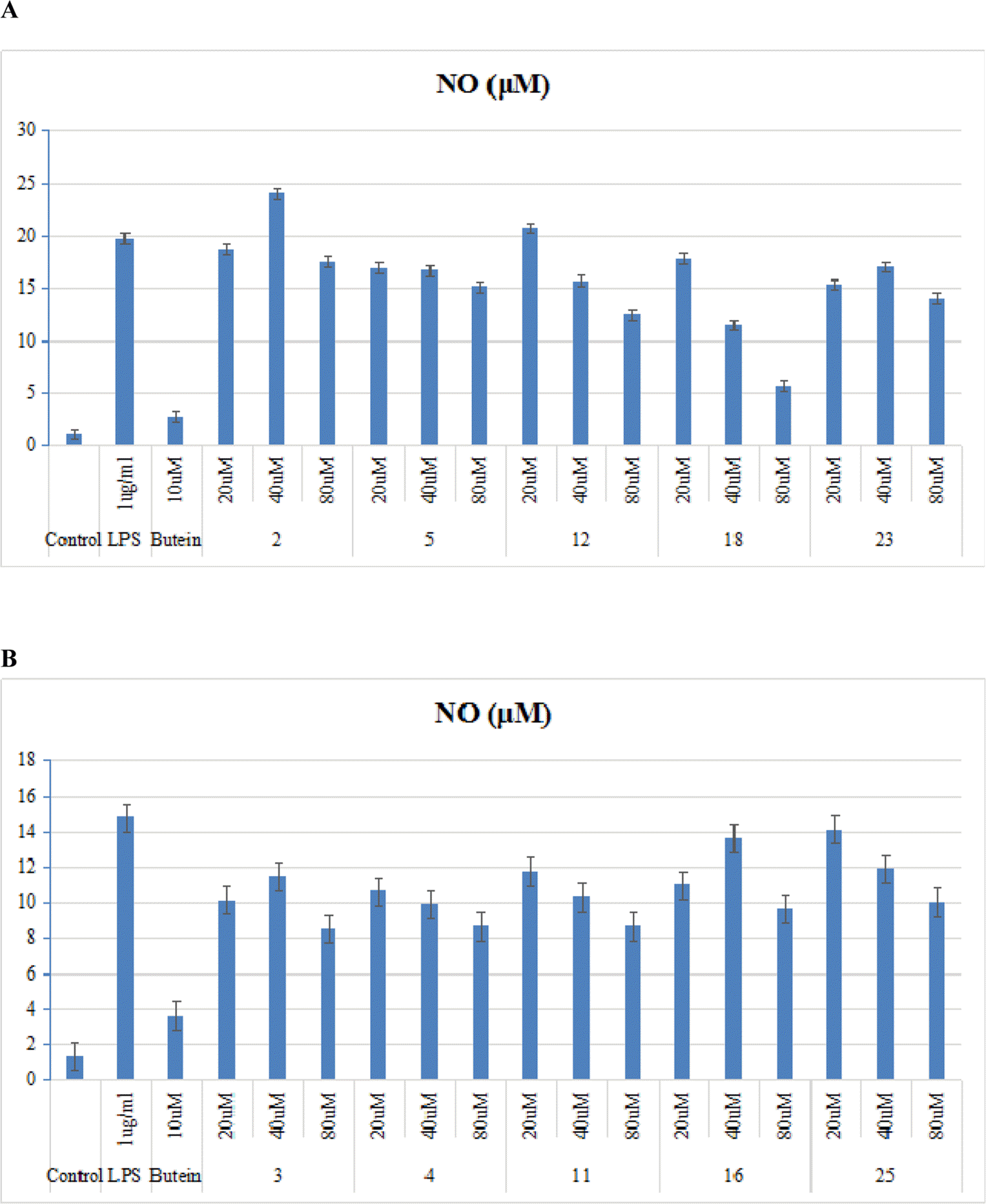 | Fig. 2.Effects of selected compounds 2-5, 11, 12, 16, 18, 23, and 25 on productions of NO in LPS-stimulated BV2 microglias. Cells were pretreated for 30 min with the indicated concentrations of selected compounds, then stimulated for 24 h with LPS (1 µg/mL). The concentrations of nitrite were determined as described in the Experimental Section. Data represent the mean values of three experiments ± SD. |
Table 1.
13 C-NMR data of triterpenoids 2-5, 9, 10, 12, 14–16, 18




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download