Abstract
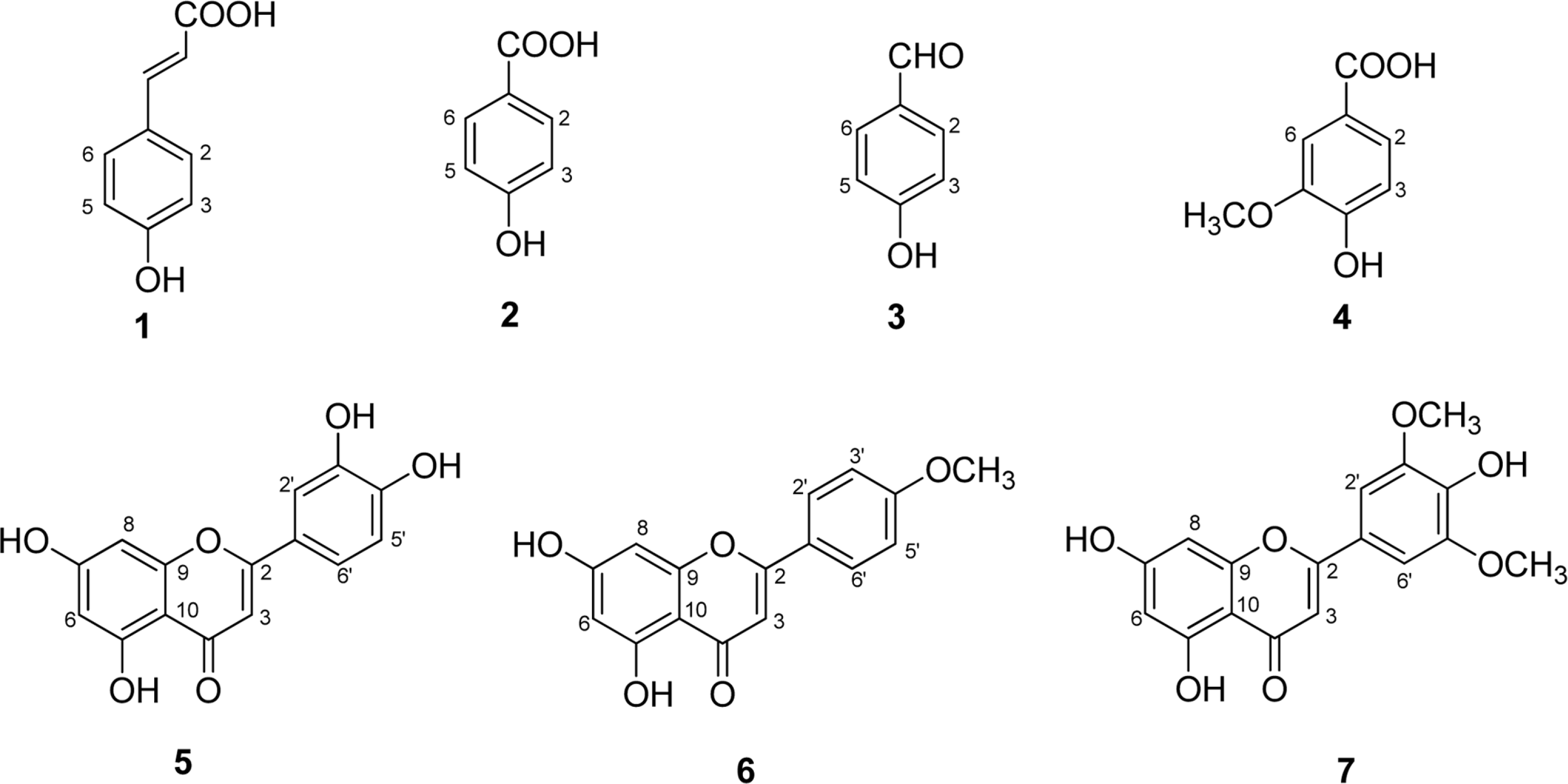
Seven phenolic compounds including p-coumaric acid (1), 4-hydroxybenzoic acid (2), 4-hydroxy-benzaldehyde (3), vanillic acid (4), luteolin (5), acacetin (6), and tricin (7), were isolated from the methylene chloride and ethyl acetate fractions of Echinochloa utilis grains. Compounds (1–4, 6) were isolated for the first time from this plant. These compounds were tested for inhibitory activities against LPS-induced NO production in RAW 264.7 cells. Compounds 5 and 6 displayed significant inhibitory effects, with IC50 values of 27.9 ± 2.6 and 14.0 ± 1.1 µM, respectively. The results suggested that E. utilis ethanolic extract may be used as a potential source of anti-inflammatory agents and functional foods for the treatment of allergic diseases.
Go to : 
REFERENCES
(1). Zedler S., Faist E.Curr. Opin. Crit. Care. 2006; 12:595–601.
(2). Mariathasan S., Monack D. M.Nat. Rev. Immunol. 2007; 7:31–40.
(3). Korhonen R., Lahti A., Kankaanranta H., Moilanen E.Curr. Drug Targets Inflamm. Allergy. 2005; 4:471–479.
(4). Salvemini D., Ischiropoulos H., Cuzzocrea S.Methods Mol. Biol. 2003; 225:291–303.
(5). Yabuno T.Econ. Bot. 1987; 41:484–493.
(6). Nozawa S., Takahashi M., Nakai H., Sato Y.Breed. Sci. 2006; 56:335–340.
(7). Park K.Y., Park R. K., Choi B. H.Korean J. Crop Sci. 1991; 36:249–253.
(8). Kim J. Y., Jang K. C., Park B. R., Han S. I., Choi K. J., Kim S. Y., Oh S. H., Ra J. E., Ha T. J., Lee J. H., Hwang J. Y., Kang H. W., Seo W. D.Food Sci. Biotechnol. 2011; 20:461–469.
(9). Watanabe M. J.Agric. Food Chem. 1999; 47:4500–4505.
(10). Migliorini P., Corradin G., Corradin S. B. J.Immunol. Methods. 1991; 139:107–114.
(11). Lee Y. J., Han J. Y., Lee C. G., Heo K., Park S. I., Park Y. S., Kim J. S., Yang K. M., Lee K. J., Kim T. H., Rhee M. H., Kim S. D. J.Ginseng Res. 2014; 38:208–214.
(12). Yang J., Wang D., Liu W., Zhang X., Bian F., Yu W.Green Chem. 2013; 15:3429–3437.
(13). Yayli N., Yildirim N., Usta A., Özkurt S., Akgün V., Turk J.Chem. 2003; 27:749–756.
(14). Xu M. L., Wang L., Hu J. H., Wang M. H. J.Food Sci. Nutr. 2009; 14:354–357.
(15). Chang S. W., Kim K. H., Lee I. K., Choi S. U., Ryu S. Y., Lee K. R.Nat. Prod. Sci. 2009; 15:234–240.
(16). Wagner H., Chari V. M., Sonnenbichler J.Tetrahedron Lett. 1976; 17:1799–1802.
(17). Shimoi K., Masuda S., Furugori M., Esaki S., Kinae N.Carcinogenesis. 1994; 15:2669–2672.
(18). Yamamoto H., Sakakibara J., Nagatsu A., Sekiya K. J.Agric. Food Chem. 1998; 46:862–865.
(19). Lin Y., Shi R., Wang X., Shen H. M.Curr. Cancer Drug Targets. 2008; 8:634–646.
(20). Seelinger G., Merfort I., Schempp C. M.Planta Med. 2008; 74:1667–1677.
(21). Park C. M., Song Y. S.Nutr. Res. Pract. 2013; 7:423–429.
(22). Yang Z. G., Jia L. N., Shen Y., Ohmura A., Kitanaka S.Molecules. 2011; 16:8305–8318.
(23). Chan T. S., Galati G., Pannala A. S., Rice-Evans C., O'Brien P. J.Free Radic. Res. 2003; 37:787–794.
(24). Pan M. H., Lai C. S., Wang Y. J., Ho C. T.Biochem. Pharmacol. 2006; 72:1293–1303.
(25). Srisook K., Srisook E., Nachaiyo W., Chan-In M., Thongbai J., Wongyoo K., Chawsuanthong S., Wannasri K., Intasuwan S., Watcharanawee K. J.Ethnopharmacol. 2015; 165:94–102.
Go to : 
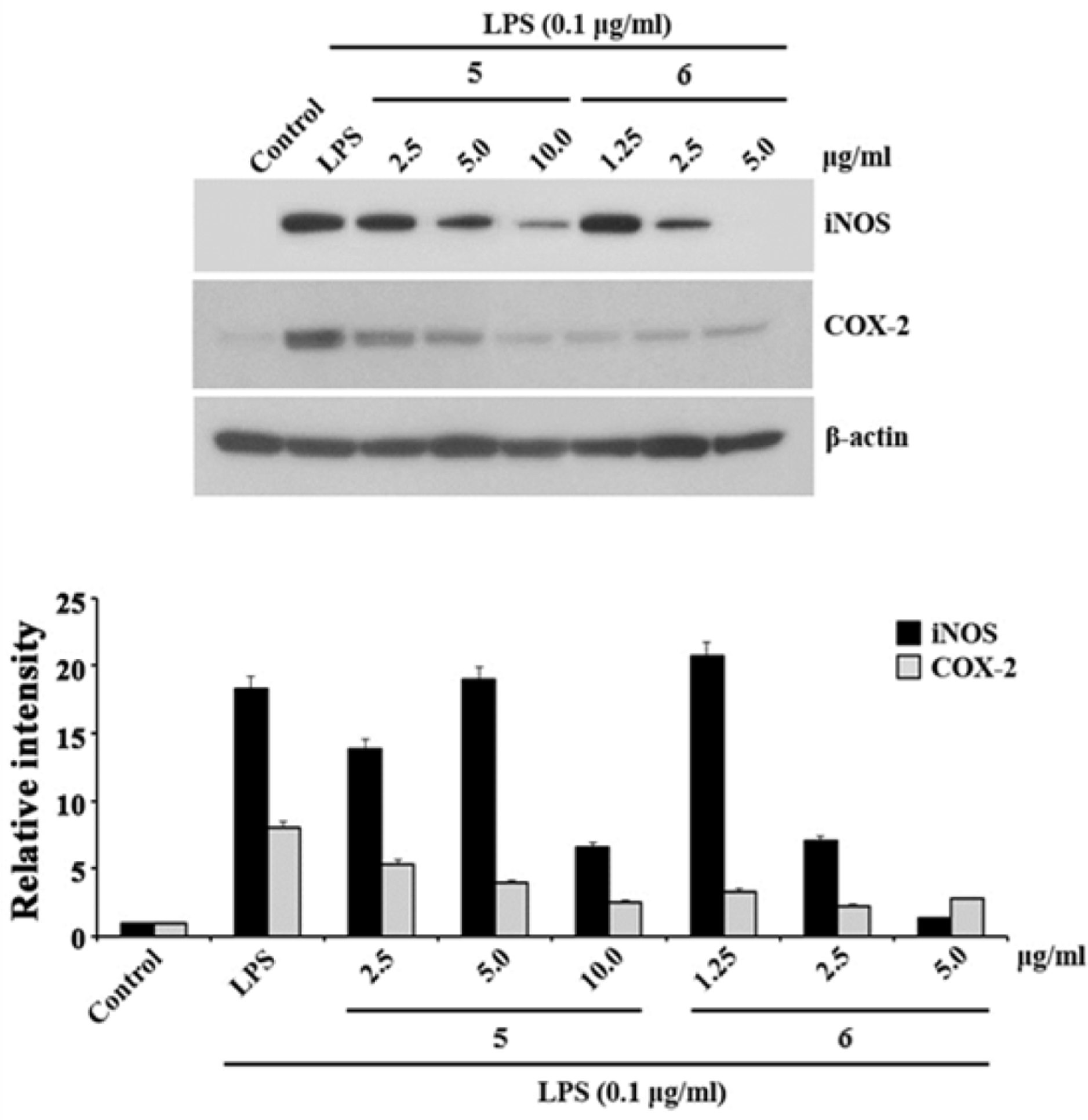 | Fig. 2.The effects of compounds 5 and 6 on LPS-induced rise of iNOS and COX-2 levels in RAW 264.7 cells. The RAW 264.7 cells were pretreated with LPS (0.1 µg/mL) for 30 min, followed by concentrations of 2.5, 5.0, and 10.0 µg/mL for 5 and 1.25, 2.5, and 5.0 µg/mL for 6 for 24 h. Whole-cell lysates were blotted with the indicated antibodies. β-Actin level was used as a loading control. The expressions of iNOS and COX-2 were assessed by Western blotting analysis using specific antibodies for individual proteins. The results are representatives of three independent experiments. |
Table 1.
Effects of IC50 values of isolated compounds 1–7 from E. utilis on LPS-stimulated NO production and cell viability.
| Compounds | NO production and cell viabilityb | |
|---|---|---|
| IC50 value (µM) | Survival rate (%) | |
| 1 | > 100 | > 99 |
| 2 | > 100 | > 99 |
| 3 | > 100 | > 99 |
| 4 | > 100 | 100 |
| 5 | 27.9 ± 2.6 | > 99 |
| 6 | 14.0 ± 1.1 | 100 |
| 7 | > 100 | > 99 |
| Dexamethasonea | 0.4 ± 0.05 | 100 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download