Abstract
In this report, we investigated the antioxidant (peroxyl radical-scavenging and reducing capacities) and anti-osteoporotic activities of extracts and isolated constituents (1–16) from the rhizomes of Kaempferia parviflora Wall. ex Baker on pre-osteoclastic RAW 264.7 cells. Compound 5 exhibited significant peroxyl radical-scavenging capacity, with TE value of 8.47 ± 0.52 µM, while compound 13 showed significant reducing capacity, with CUPRAC value of 5.66 ± 0.26 µM, at 10.0 µM. In addition, flavonoid compounds 2, 4, 6, 8, 10, 12, and terpene compound 15 showed significant inhibition of tartrate-resistant acid phosphatase (TRAP) in NF-κB ligand-induced osteoclastic RAW 264.7 cells, with values ranging from 16.97 ± 1.02 to 64.67 ± 2.76%. These results indicated that K. parviflora could be excellent sources for the antioxidant and anti-osteoporotic traditional medicinal plants.
Go to : 
REFERENCES
(1). Hu F. B., Willett W. C. J.Am. Med. Assoc. 2002; 288:2569–2578.
(2). Pietta P. G. J.Nat. Prod. 2000; 63:1035–1042.
(3). Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M.Nature. 1999; 397:315–323.
(4). Yenjai C., Prasanphen K., Daodeeb S., Wongpanich V., Kittakoop P.Fitoterapia. 2004; 75:89–92.
(5). Sutthanut K., Sripanidkulchai B., Yenjai C., Jay M. J.Chromatogr. A. 2007; 1143:227–233.
(6). Chaipech S., Morikawa T., Ninomiya K., Yoshikawa M., Pongpiriyadacha Y., Hayakawa T., Muraoka O.Chem. Pharm. Bull. 2012; 60:62–69.
(7). Azuma T., Kayano S. I., Matsumura Y., Konishi Y., Tanaka Y., Kikuzaki H.Food Chem. 2011; 125:471–475.
(8). Sawasdee P., Sabphon C., Sitthiwongwanit D., Kokpol U.Phytother. Res. 2009; 23:1792–1794.
(9). Patanasethanont D., Nagai J., Yumoto R., Murakami T., Sutthanut K., Sripanidkulchai B. O., Yenjai C., Takano M. J.Pharma. Sci. 2007; 96:223–233.
(10). Wattanapitayakul S. K., Suwatronnakorn M., Chularojmontri L., Herunsalee A., Niumsakul S., Charuchongkolwongse S., Chansuvanich N. J.Ethnopharmacol. 2007; 110:559–562.
(11). Akase T., Shimada T., Terabayashi S., Ikeya Y., Sanada H., Aburada M. J.Nat. Med. 2011; 65:73–80.
(12). Wong C. S., Tansakul P., Tewtrakul S. J.Ethnopharmacol. 2009; 124:576–580.
(13). Tewtrakul S., Subhadhirasakul S., Kummee S. J.Ethnopharmacol. 2008; 116:191–193.
(14). Wattanapitayakul S. K., Chularojmontri L., Herunsalee A., Charuchongkolwongse S., Chansuvanich N.Fitoterapia. 2008; 79:214–216.
(15). Patanasethanont D., Nagai J., Matsuura C., Fukui K., Sutthanut K., Sripanidkulchai B.O., Yumoto R., Takano M.Eur. J. Pharmacol. 2007; 566:67–74.
(16). Yenjai C., Wanich S.Bioorg. Med. Chem. Lett. 2010; 20:2821–2823.
(17). Aruoma O. I., Deiana M., Jenner A., Halliwell B., Kaur H., Banni S., Corongiu F. P., Dessí M. A., Aeschbach R. J.Agric. Food Chem. 1998; 46:5181–5187.
(18). Kurihara H., Fukami H., Asami S., Toyoda Y., Nakai M., Shibata H., Yao X. S.Biol. Pharm. Bull. 2004; 27:1093–1098.
(19). van de Loosdrecht A. A., Beelen R. H. J., Ossenkoppele G. J., Broekhoven M. G., Langenhuijsen M. M. A.C. J. Immunol. Methods. 1994; 174:311–320.
(20). Lee S. H., Ding Y., Yan X. T., Kim Y. H., Jang H. D. J.Nat. Prod. 2013; 76:615–620.
(21). Vichitphan S., Vichitphan K., Sirikhansaeng P.KMITL Sci. Tech. J. 2007; 7:97–105.
(22). Horikawa T., Shimada T., Okabe Y., Kinoshita K., Koyama K., Miyamoto K. I., Ichinose K., Takahashi K., Aburada M.Biol. Pharm. Bull. 2012; 35:686–692.
Go to : 
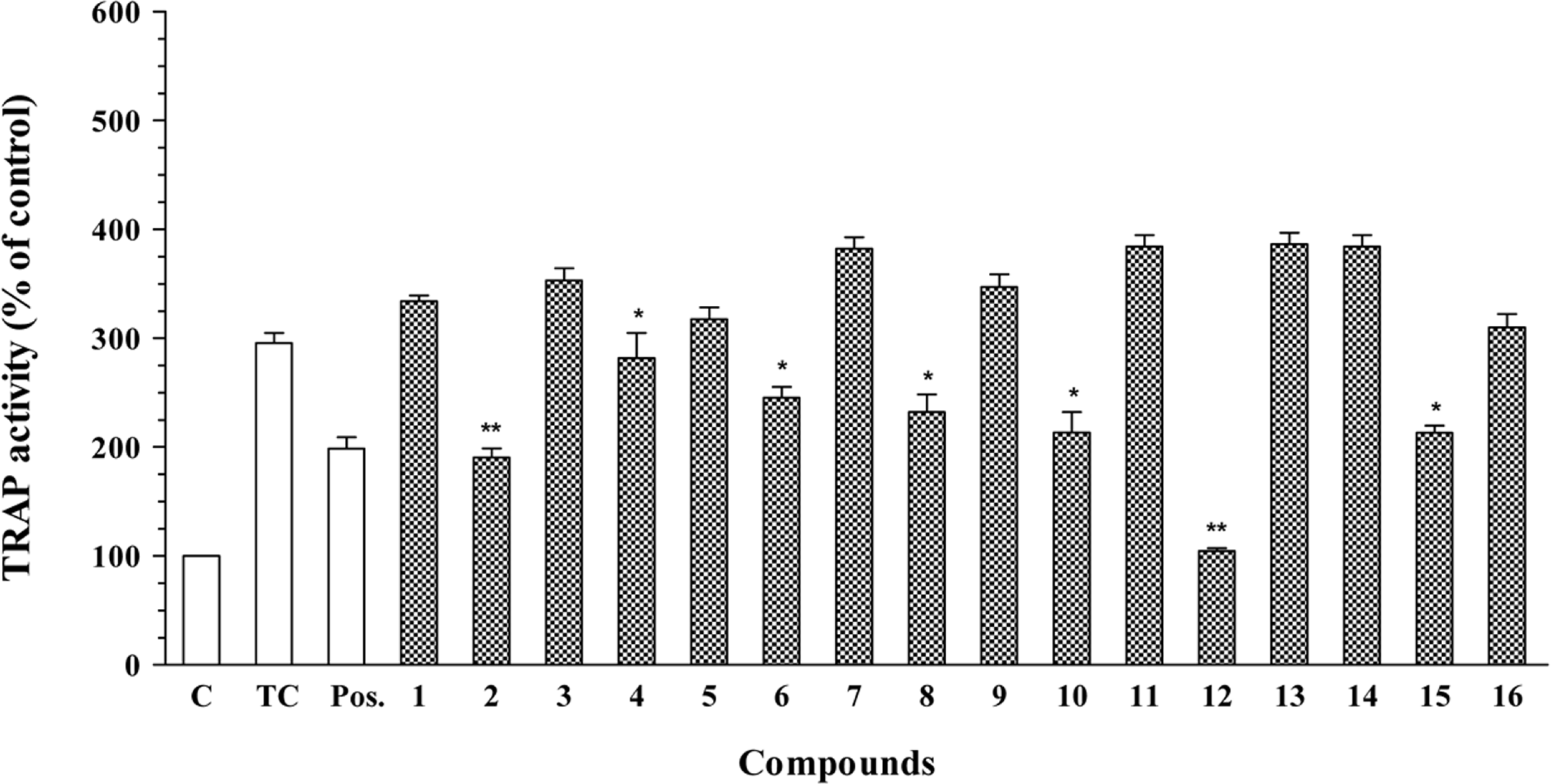 | Fig. 2.Inhibitory effects of compounds 1–16 on TRAP activity in RANKL-induced osteoclastic RAW 264.7 cells. TRAP activity was measured from cultures after 5 days of treatment with RANKL and test compounds (10.0 µM). The treated control was obtained from RAW 264.7 cells cultured with RANKL stimulation and without test compounds. Data are expressed as percentages of the treated control (mean ± SD, n = 3,∗P < 0.05 and∗∗P < 0.01 vs TC. C: control, which was not treated; TC: treated control, which was treated with RANKL). Daidzein (10.0 µM) was use as a positive control (Pos.). |
Table 1.
Antioxidant activities of the extracts and isolated compounds (1–16) from K. parviflora




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download