Abstract
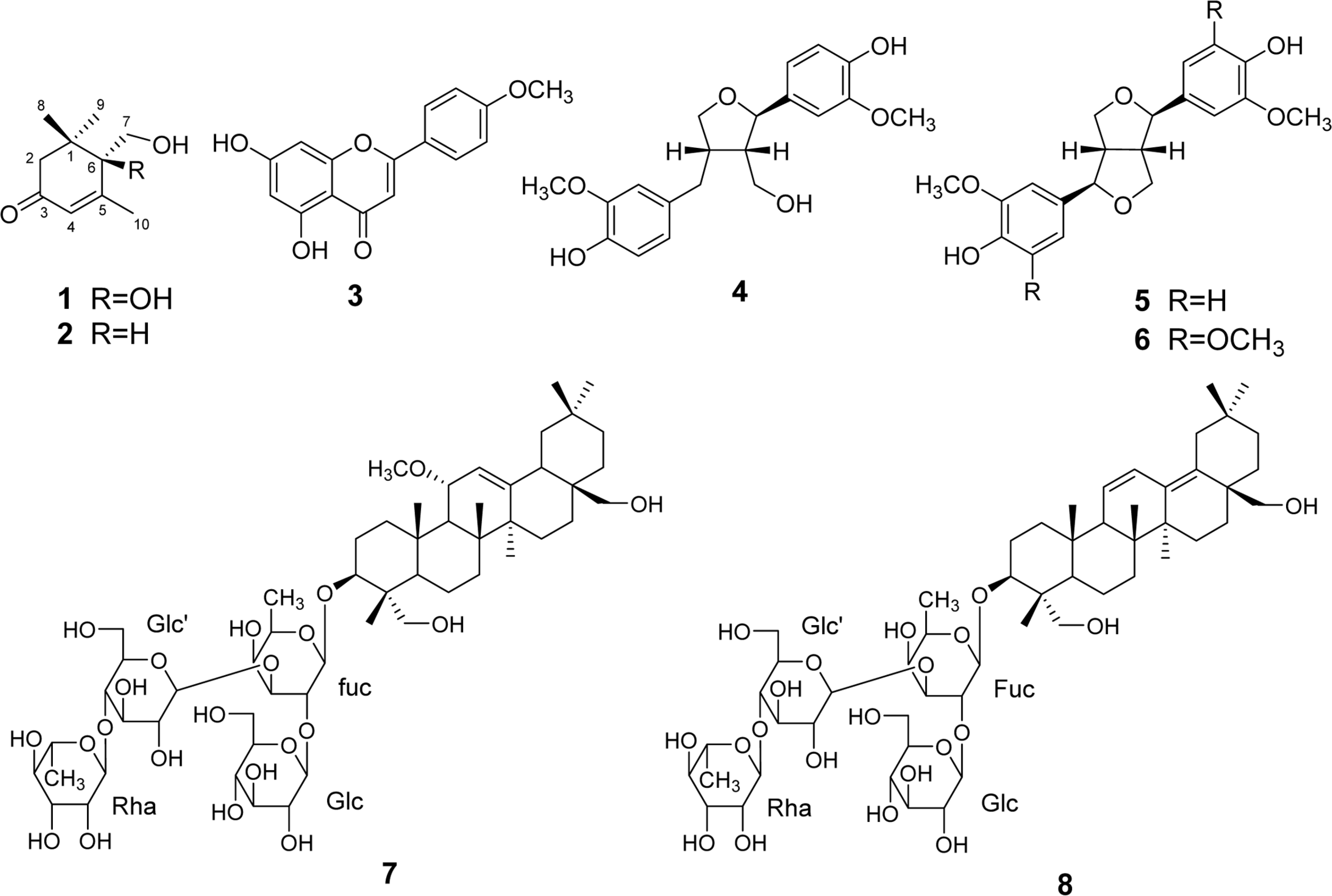
Bioactivity-guided fractionation of a methanolic extract of Buddleja officinalis led to the isolation of two monoterpenes, crocusatin M (1), crocusatin C (2), a flavonoid, acacetin (3), three lignans, lariciresinol (4), pinoresinol (5), and syringaresinol (6), and two triterpenoidal saponins, mimengoside B (7) and songarosaponin A (8). The structures of isolates were identified based on 1D-, 2D-NMR, and MS data analysis. All isolates were tested for their inhibition on LPS-induced NO production in RAW 264.7 cells. As a result, mimengoside B (7) and songarosaponin A (8) showed a mild inhibitory activity of NO production.
Go to : 
REFERENCES
(1). Tang W., Eisenbrand G.Handbook of Chinese Medicinal Plants; Wiley-VCH: Germany. 2011; 267–270.
(3). Tai B. H., Nhiem N. X., Quang T. H., Ngan N. T., Tung N. H., Kim Y., Lee J. J., Myung C. S., Cuong N. M., Kim Y. H.Bioorg. Med. Chem. Lett. 2011; 21:3462–3466.
(4). Tai B. H., Jung B. Y., Cuong N. M., Linh P. T., Tung N. H., Nhiem N. X., Huong T. T., Anh N. T., Kim J. A., Kim S. K., Kim Y. H.Biol. Pharm. Bull. 2009; 32:1952–1956.
(5). Guo H., Koike K., Li W., Satou T., Guo D., Nikaido T. J.Nat. Prod. 2004; 67:10–13.
(6). Piao M. S., Kim M. R., Lee D. G., Park Y., Hahm K. S., Moon Y. H., Woo E. R.Arch. Pharm. Res. 2003; 26:453–457.
(7). Liao Y. H., Houghton P. J., Hoult J. R. J.Nat. Prod. 1999; 62:1241–1245.
(8). Lee C., Lee S., Park S. Y.Nat. Prod. Sci. 2013; 19:355–359.
(9). Li C. Y., Wu T. S.Chem. Pharm. Bull. 2002; 50:1305–1309.
(10). Yim S. H., Kim H. J., Lee I. S.Arch. Pharm. Res. 2003; 26:128–131.
(11). Lee D. Y., Song M. C., Yoo K. H., Bang M. H., Chung I. S., Kim S. H., Kim D. K., Kwon B. M., Jeong T. S., Park M. H., Baek N. I.Arch. Pharm. Res. 2007; 30:402–407.
(12). Kwon H. C., Choi S. U., Lee J. O., Bae K. H., Zee O. P., Lee K. R.Arch. Pharm. Res. 1999; 22:417–422.
(13). Emam A. M., Moussa A. M., Faure R., Elias R., Balansard G. J.Ethnopharmacol. 1997; 58:215–217. 1992.
(14). Ding N., Yahara S., Nohara T.Chem. Pharm. Bull. 1992; 40:780–782.
(15). Seifert K., Preiss A., Johne S., Schmidt J., Lien N. T., Lavaud C., Massiot G.Phytochemistry. 1991; 30:3395–3400.
(16). Tundis R., Bonesi M., Deguin B., Loizzo M. R., Menichini F., Conforti F., Tillequin F., Menichini F.Bioorg. Med. Chem. 2009; 17:4542–4547.
(17). Jung H. J., Kim S. G., Nam J. H., Park K. K., Chung W. Y., Kim W. B., Lee K. T., Won J. H., Choi J. W., Park H. J.Biol. Pharm. Bull. 2005; 28:1668–1671.
(18). Won J. H., Im H. T., Kim Y. H., Yun K. J., Park H. J., Choi J. W., Lee K. T. Br. J.Pharmacol. 2006; 148:216–225.
(19). Oh W. J., Jung U., Eom H. S., Shin H. J., Park H. R.Molecules. 2013; 18:9195–9206.
Go to : 
Table 1.
13 C NMR data of compounds 7 and 8 (δ in ppm, 125MHz, CD3 OD)a
Table 2.
Inhibitory effects of compounds 1–8 on LPS-induced NO production
| compound | IC50 (μ M)a |
|---|---|
| crocusatin M (1) | >50 |
| crocusatin C (2) | >50 |
| acacetin (3) | >50 |
| lariciresinol (4) | >50 |
| pinoresinol (5) | >50 |
| syringaresinol (6) | >50 |
| mimengoside B (7) | 22.1 ± 0.8 |
| songarosaponin A (8) | 25.6 ± 1.0 |
| aminoguanidineb | 17.5 ± 0.7 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download