Abstract
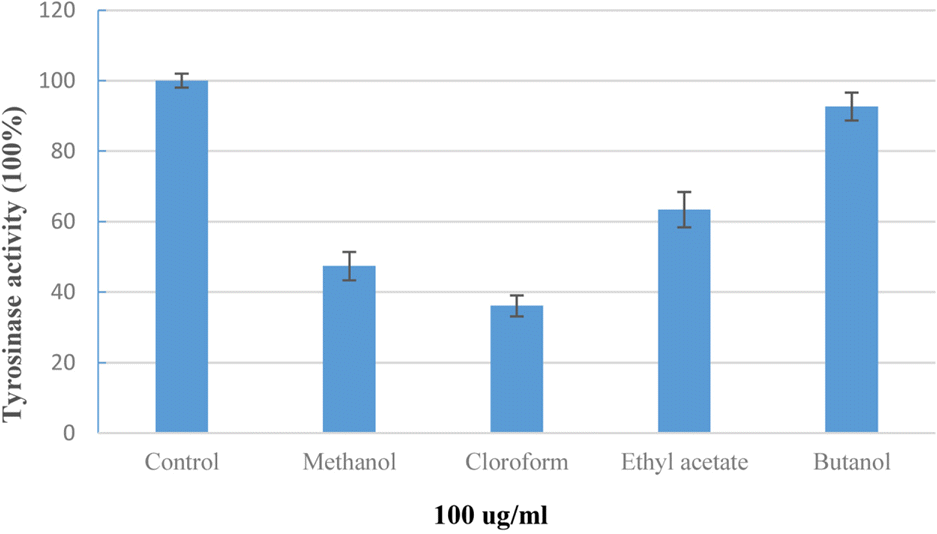
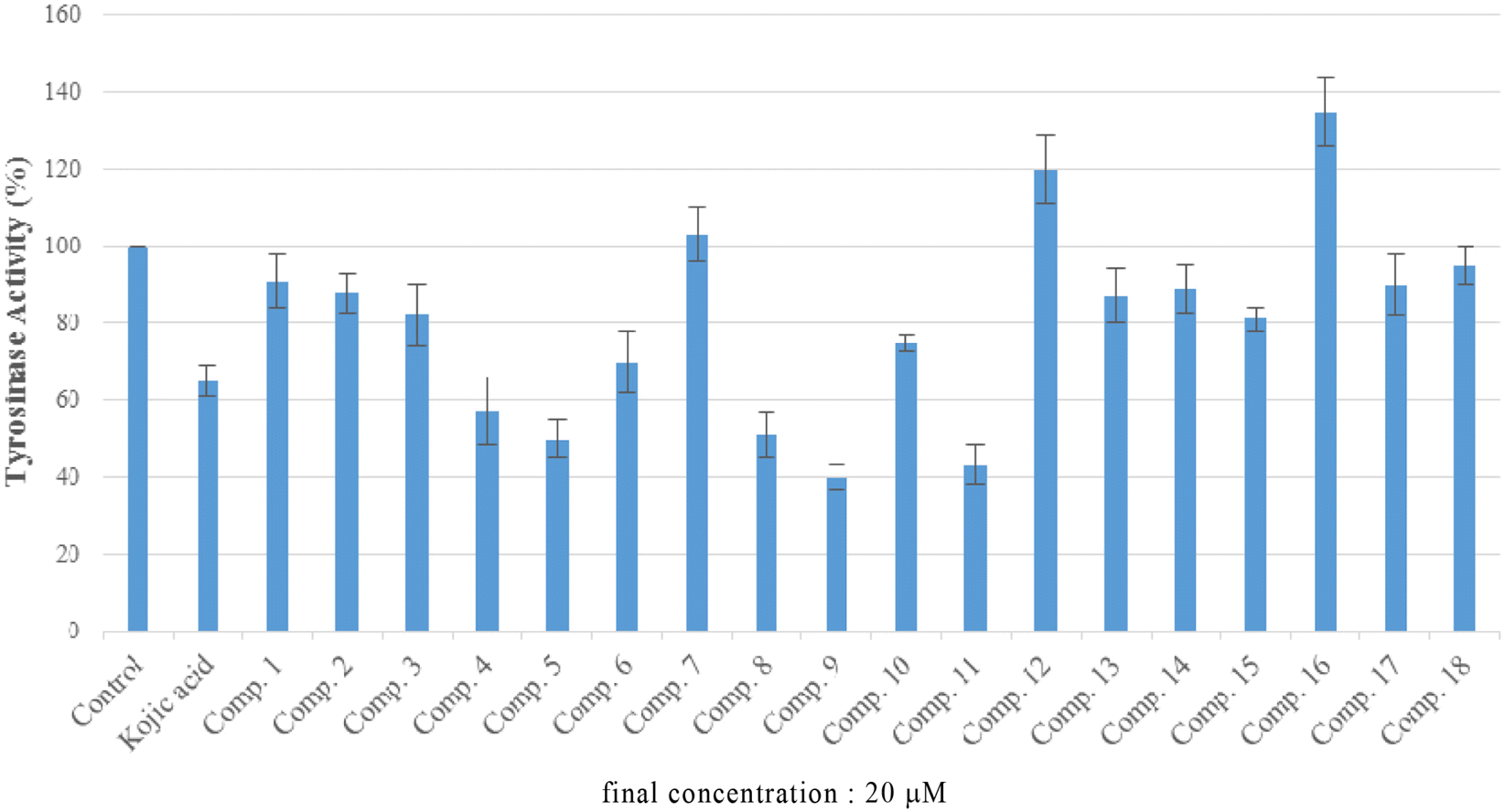
Phytochemical investigation of the stems of Pueraria lobata (Wild) Ohwi (Leguminosae), led to the isolation of eighteen known compounds: β-amyrone (1), (+)-pinoresinol (2), (+)-syringaresinol (3) (+)-syringaresinol-O-β-D-glucoside (4), (+)-lariciresinol (5), (−)-tuberosin (6), naringenin (7), liquiritigenin (8), isoliquiritigenin (9) genistein (10), daidzein (11) daidzin (12) daidzein 4',7-diglucoside (13) 2,4,4'-trihydroxy deoxybenzoin (14), S-(+)-1-hydroxy-3-(4-hydroxyphenyl)-1-(4-hydroxy-2-methoxy-phenyl)propan-2-one (15), methyl 2-O-β-D-glucopyra-nosylbenzoate (16), pyromeconic acid 3-O-β-D-glucopyranoside 6'- (O-4″-hydroxy-3-methoxybenzoate) (17), and allantion (18). The chemical structures of these compounds were elucidated from spectroscopic data and by comparison of those data with previously published results. The effects of isolated compounds on mushroom tyrosinase enzymatic activity were screened. The results indicated that, chloroform extract of P. lobata stems turned out to be having tyrosinase inhibitory effect, and only compounds 5, 8, 9, and 11 showed enzyme inhibitory activity, with IC50 values of 21.49 ± 4.44, 25.24 ± 6.79, 4.85 ± 2.29, and 17.50 ± 1.29 μM, respectively, in comparison with these of positive control, kojic acid (IC50 12.28 ± 2.72 μ M). The results suggest that P. lobata stems extract as well as its chemical components may represent as potential candidates for tyrosinase inhibitors.
Go to : 
REFERENCES
(1). Seo S. Y., Sharma V. K., Sharma N. J.Agric. Food Chem. 2003; 51:2837–2853.
(2). Nhiem N. X., Yen H. T., Luyen B. T. T., Tai B. H., Hoan P. V., Thao N. P., Anh H. L. T., Ban N. K., Kiem P. V., Minh C. V., Kim J. H., Jeon M. N., Kim Y. H.Bull. Korean Chem. Soc. 2015; 36:703–706.
(3). Cho Y. J., Son B. W., Jeong D. Y., Choi H. D., Park J. H. Kor. J.Pharmacogn. 1998; 29:193–197.
(4). Chen T. R., Shih S. C., Ping H. P., Wei Q. K. J.Food Drug Anal. 2012; 20:681–685.
(5). Hung V. P., Morita N.Food Chem. 2007; 105:749–755.
(6). Li G., Zhang Q., Wang Y.Zhongguo Zhong Yao Za Zhi. 2010; 35:3156–3160.
(7). Luo X. D., Wu S. H., Ma Y. B., Wu D. G.Acta Bot. Sin. 2001; 43:426–430.
(8). Xie L. H., Akao T., Hamasaki K., Deyama T., Hattori M.Chem. Pharm. Bull. 2003; 51:508–515.
(9). Park J. A., Kim H. J., Jin C. B., Lee K. T., Lee Y. S.Arch. Pharm. Res. 2003; 26:1009–1013.
(10). Shahat A. A., Abdel-Azim N. S., Pieters L., Vlietinck A. J.Fitoterapia. 2004; 75:771–773.
(11). Wang Q. H., Peng K., Tan L. H., Dai H. F.Molecules. 2010; 15:4011–4016.
(12). Shirataki Y., Tsuzuku T., Yokoe I., Hirano R. T., Komatsu M.Chem. Parm. Bull. 1990; 38:1712–1716.
(13). Kulesh N. I., Vasilevskaya N. A., Veselova M. V., Denisenko V. A., Fedoreev S. A.Chem. Nat. Compd. 2008; 44:712–714.
(14). Yahara S., Ogata T., Saijo R., Konishi R., Yamahara J., Miyahara K., Nohara T.Chem. Pharm. Bull. 1989; 37:979–987.
(15). Veitch N. C., Sutton P. S., Kite G. C., Ireland H. E. J.Nat. Prod. 2003; 66:210–216.
(16). Kim B. H., Kim C. M. Kor. J.Pharmacogn. 1995; 26:18–22.
(17). Yang M. C., Kim D. S., Jeong S. W., Ma J. Y.Korean J. Medicinal Crop. Sci. 2011; 19:446–455.
(18). Jun M., Fu H.-Y., Hong J., Wan X., Yang C. S., Ho C. T. J.Food Sci. 2003; 68:2117–2122.
(19). Kinjo J. E., Furusawa J. I., Baba J., Takeshita T., Yamasaki M., Ohara T.Chem. Pharm. Bull. 1987; 35:4846–4850.
(20). Ng L. T., Ko H. H., Lu T. M.Bioorg. Med. Chem. 2009; 17:4360–4366.
(21). Bezuidenhout S. C., Bezuidenhoudt B. C. B., Ferreira D.Phytochemistry. 1988; 27:2329–2334.
(22). Wang C., Zhang T. T., Du G. H., Zhang D. M. J.Asian Nat. Prod. Res. 2011; 13:817–825.
(23). Chai X., Su Y. F., Guo L. P., Wu D., Zhang J. F., Si C. L., Kim J. K., Bae Z. S.Biochem. Syst. Ecol. 2008; 36:216–218.
(24). Yin F., Hu L., Pan R.Chem. Pharm. Bull. 2004; 52:1440–1444.
(25). Khatib S., Nerya O., Musa R., Shmuel M., Tamir S., Vaya J.Bioorg. Med. Chem. 2005; 13:433–441.
(26). Wang Y., Curtis-Long M. J., Lee B. W., Yuk H. J., Kim D. W., Tan X. F., Park K. H.Bioorg. Med. Chem. 2014; 22:1115–1120.
(27). Kim N. K., Park H. M., Lee J. K., Ku K. M., Lee C. H. J.Agric. Food Chem. 2015; 63:8631–8639.
Go to : 
Table 1.
In vitro tyrosinase inhibitory activity of compounds 5, 8,9 and 11
| Compound | IC50 a (mM) |
|---|---|
| (+)-lariciresinol (5) | 21.49 ± 4.44 |
| liquiritigenin (8) | 25.24 ± 6.79 |
| isoliquiritigenin (9) | 4.85 ± 2.29 |
| daidzein (11) | 17.5 ± 1.29 |
| Kojic acidb | 12.27 ± 2.72 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download