Abstract
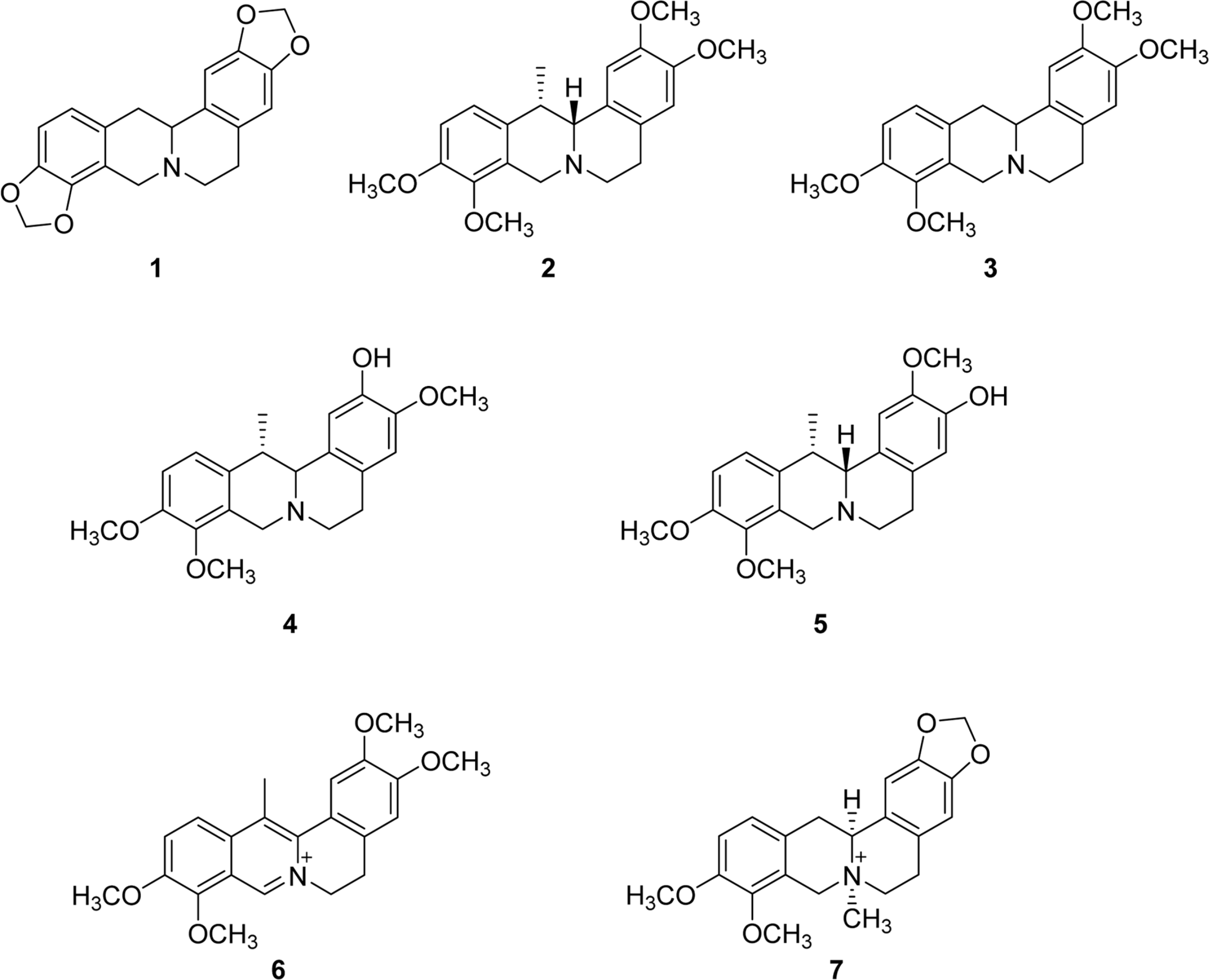
A methanolic extract of Corydalis ternata having aldose reductase inhibitory activity was examined as a possible aldose reductase (ALR2) inhibitor, a key enzyme involved in diabetic complications. Seven alkaloids, tetrahydrocoptisine (1), corydaline (2), tetrahydropalmatine (3), isocorybulbine (4), corybulbine (5), dehydrocory-daline (6), and N-methyltetrahydroberbinium (7) were isolated from CHCl3 fraction of C. ternata methanol extract. Among them, compounds 1, 5, and 7 exhibited 5.04 ± 1.97%, 5.00 ± 1.26%, and 1.80 ± 2.33% inhibitions, respectively at 40 µ M. The activities of the single compounds were not comparable to that of the whole extract, suggesting that the whole combination of each single compound was responsible for the activity of the extract as shown in many cases of natural medicines. Even though this is the second report on aldose reductase inhibition activity of C. ternata, recombinant human aldose reductase was employed in this study unlike in the previous report. Furthermore, the aldose reductase inhibitory activities of isocorybulbine, corybulbine, and N-methy-ltetrahydroberbinium, to the best of our knowledge, were evaluated for the first time in this study. These results suggest a use of the extract of C. ternata for ameliorating diabetic complications.
Go to : 
REFERENCES
(1). Lee H. Y., Kim C. W. Kor. J.Pharmacogn. 1999; 30:332–334.
(2). Kubo M., Matsuda H., Tokuoka K., Ma S., Shiomoto H.Biol. Pharm. Bull. 1994; 17:262–265.
(3). Kim S. R., Hwang S. Y., Jang Y. P., Park M. J., Markelonis G. J., Oh T. H., Kim Y. C.Planta Med. 1999; 65:218–221.
(4). Lin M. T., Chueh F. Y., Hsieh M. T.Neurosci. Lett. 2001; 315:53–56.
(5). Liu G. Q., Algeri S., Garattini S.Arch. Int. Pharmacodyn. Ther. 1982; 258:39–50.
(6). Chang C. K., Chueh F. Y., Hsieh M. T., Lin M. T.Neurosci. Lett. 1999; 267:109–112.
(7). Sharma O. P.Plant Taxonomy 2 nd Ed; Tata McGraw-Hill company: New Delhi. 2009; 238.
(8). Cheng X., Ni B., Zhang Z., Liu Q., Wang L., Ding Y., Hu Y.Connect. Tissue Res. 2013; 54:118–122.
(9). Muppalaneni N. B., Rao A. A.Bioinformation. 2012; 8:980–983.
(10). Chatzopoulou M., Alexiou P., Kotsampasakou E., Demopoulos V. J.Expert Opin. Ther. Pat. 2012; 22:1303–1323.
(11). Ramana K. V.Biomol. Concepts. 2011; 2:103–114.
(12). Obrosova I. G., Kador P. F.Curr. Pharm. Biotechnol. 2011; 12:373–385.
(13). Lorenzi M.Exp. Diabetes Res. 2007; 2007:61038.
(14). Anil Kumar P., Bhanuprakash Reddy G.Exp. Eye Res. 2007; 85:739–740.
(15). de la Fuente J. A., Manzanaro S.Nat. Prod. Rep. 2003; 20:243–251.
(16). Pitts N. E., Vreeland F., Shaw G. L., Peterson M. J., Mehta D. J., Collier J., Gundersen K.Metabolism. 1986; 35:96–100.
(17). Pfeifer M. A., Schumer M. P., Gelber D. A.Diabetes. 1997; 46:S82–S89.
(18). Matsuda H, Morikawa T., Toguchida I., Yoshikawa M.Chem. Pharm. Bull. 2002; 50:788–795.
(19). Matsuda H, Wang T., Managi H., Yoshikawa M.Bioorg. Med. Chem. 2003; 11:5317–5323.
(20). Fernández M, Caballero J., Helguera A. M., Castro E. A., González M. P.Bioorg. Med. Chem. 2005; 13:3269–3277.
(21). Kubo M., Matsuda H., Tokuoka K., Kobayashi Y., Ma S., Tanaka T.Biol. Pharm. Bull. 1994; 17:458–459.
(22). Sato S., Kador P. F.Biochem. Pharmacol. 1990; 40:1033–1042.
(23). Yu L. L., Li R. T., Ai Y. B., Liu W., Deng Z. S., Zou Z. M.Molecules. 2014; 19:13332–13341.
(24). Likhitwitayawuid K., Ruangrungsi N., Geoffrey A., Cordell G. A. J.Sci. Soc. Thailand. 1993; 19:87–96.

(25). Cushman M., Dekow F. W.Tetrahedron. 1978; 34:1435–1439.
(26). Hughes D. W., Holland H. L., MacLean D. B. Can. J.Chem. 1976; 54:2252–2260.
Go to : 
Table 1.
Effects of methanolic extracts and fractions of Corydalis tuber on rhALR2
| Ex./Frs. | Conc. (µ g/ml) | Inhibition1 (%) |
|---|---|---|
| MeOH ex. | 100 | 75.4 ± 5.2 |
| 50 | 32.5 ± 2.1 | |
| 25 | 11.4 ± 1.3 | |
| CHCl3 fr. | 100 | 63.4 ± 4.2 |
| 50 | 25.8 ± 3.4 | |
| 25 | 58.7 ± 1.1 | |
| EtOAc fr. | 100 | 57.5 ± 1.6 |
| 50 | −2 | |
| 25 | −2 | |
| Water fr. | 100 | −2 |
Table 2.
Inhibitory effect of compounds isolated from C. ternata on rhALR2 activity
| Compounds | Conc. (µ M) | Inhibition2 (%) | IC503 (µ M) |
|---|---|---|---|
| TMG1 | 40 | 72.62 ± 0.10 | |
| 20 | 62.56 ± 1.28 | ||
| 10 | 51.38 ± 1.43 | 10.06 | |
| 55 | 36.74 ± 2.20 | ||
| 1 | 40 | 55.04 ± 1.97 | >40 |
| 2 | 40 | −4 | >40 |
| 3 | 40 | −4 | >40 |
| 4 | 40 | −4 | >40 |
| 5 | 40 | 55.00 ± 1.26 | >40 |
| 6 | 40 | −4 | >40 |
| 7 | 40 | 51.80 ± 2.33 | >40 |
2 Inhibitory rate was calculated as a percentage relative to the control value and expressed as the mean ± standard deviation of triplicate experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download