Abstract
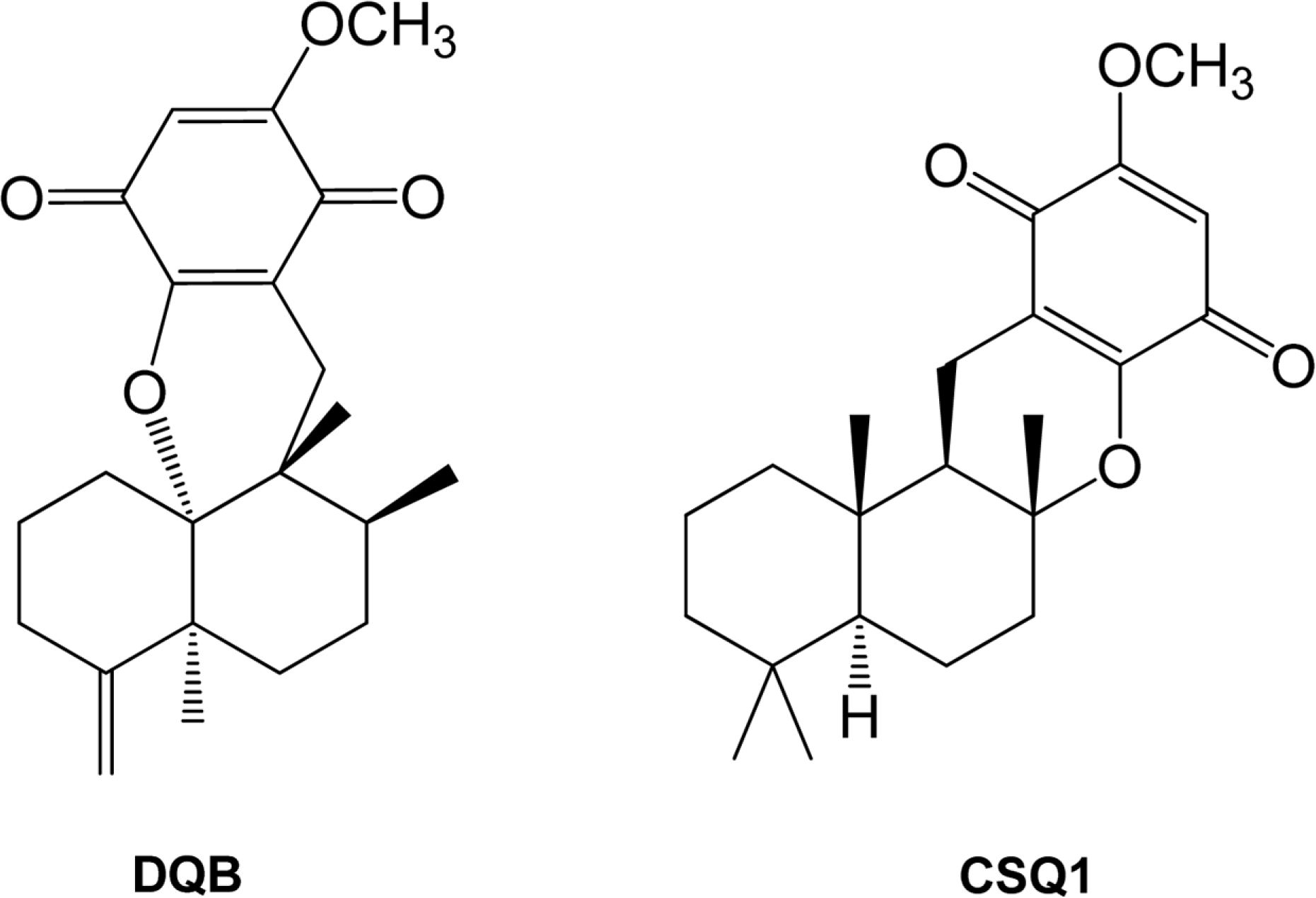
Sesquiterpene-quinone is a class of secondary metabolites frequently encountered from marine sponge. The present study was designed to examine the anti-inflammatory action of sponge-derived dactyloquinone B (DQB) and cyclospongiaquinone-1 (CSQ1) mixture using lipopolysaccharide (LPS)-induced inflammatory responses. We measured the production of nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) and expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein. TNF-α, IL-1β, and IL-6 production, which increased by treatment with LPS, were significantly inhibited by DQB and CSQ1 mixture. It also decreased the production of NO production, and iNOS and COX-2 expression. Furthermore, it reduced 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced ear edema of ICR mice. These results demonstrate that sesquiterpene-quinone, DQB and CSQ1 mixture, might serve as a chemical pipeline for the development of anti-inflammatory agent.
Go to : 
REFERENCES
(1). Blunt J. W., Copp B. R., Keyzers R. A., Munro M. H., Prinsep M. R.Nat. Prod. Rep. 2014; 31:160–258.
(2). Marcos I. S., Conde A., Moro R. F., Basabe P., Diez D., Urones J. G.Mini Rev. Org. Chem. 2010; 7:230–254.
(3). Luibrand R. T., Erdman T. R., Vollmer J. J., Scheuer P. J., Finer J., Clardy J.Tetrahedron. 1979; 35:609–612.
(4). Capon R. J., MacLeod J. K. J.Org. Chem. 1987; 52:5059–5060.
(5). Takizawa P. A., Yucel J. K., Veit B., Faulkner D. J., Deerinck T., Soto G., Ellisman M., Malhotra V.Cell. 1993; 73:1079–1090.
(6). Kazlauskas R., Murphy P. T., Warren R. G., Wells R. J., Blount J. F.Aust. J. Chem. 1978; 31:2685–2697.
(7). Fernandez A., Alvarez E., Alvarez-Manzaneda R., Chahboun R., Alvarez-Manzaneda E.Chem. Comm. 2014; 50:13100–13102.
(8). Mitome H., Nagasawa T., Miyaoka H., Yamada Y., van Soest R. W. J.Nat. Prod. 2001; 64:1506–1508.
(9). Mitome H., Nagasawa T., Miyaoka H., Yamada Y., van Soest R. W.Tetrahedron. 2002; 58:1693–1696.
(10). Mitome H., Nagasawa T., Miyaoka H., Yamada Y., van Soest R. W. J.Nat. Prod. 2003; 66:46–50.
(11). Ovenden S. P., Nielson J. L., Liptrot C. H., Willis R. H., Tapiolas D. M., Wright A. D., Motti C. A. J.Nat. Prod. 2011; 74:65–68.
(12). Jankam A., Somerville M. J., Hooper J. N. A., Brecknell D. J., Suksamrarn A., Garson M. J.Tetrahedron. 2007; 63:1577–1582.
(13). Lee D. S., Jeong G. S., Li B., Park H., Kim Y. C.Int. Immunopharmacol. 2010; 10:850–858.
(14). Lee D. S., Jang J. H., Ko W., Kim K. S., Sohn J. H., Kang M. S., Ahn J. S., Kim Y. C., Oh H.Mar. Drugs. 2013; 11:1409–1426.
(15). Hwang I. H., Oh J., Zhou W., Park S., Kim J.-H., Chittiboyina A. G., Ferreira D., Song G. Y., Oh S., Na M., Hamann M. T. J.Nat. Prod. 2015; 78:453–461.
(16). Agut J., Tarrida N., Sacristan A., Ortiz J. A.Methods Find. Exp. Clin. Pharmacol. 1996; 18:233–234.
(17). Liebel F., Lyte P., Garay M., Babad J., Southall M. D.Arch. Dermatol. Res. 2006; 298:191–199.
(18). Rao T. S., Currie J. L., Shaffer A. F., Isakson P. C.Inflammation. 1993; 17:723–741.
Go to : 
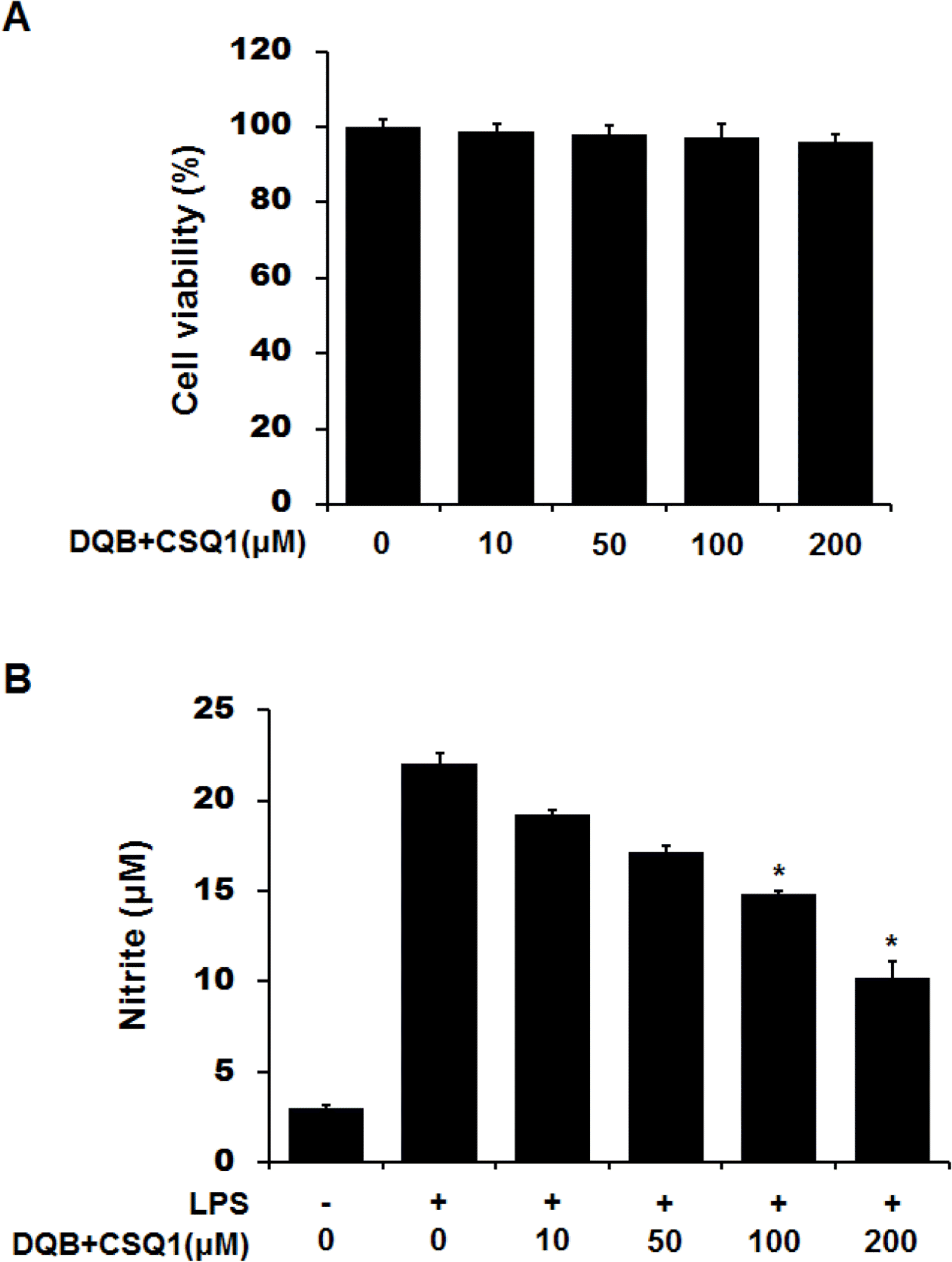 | Fig. 2.Effect of DQB and CSQ1 mixture on cell viability or NO production in RAW 264.7 cells. Cells were plated and cultured for 24 h in the absence or presence of different concentration of DQB and CSQ1 mixture (A). Cells were treated with DQB and CSQ1 mixture and LPS (100 ng/ml) for 24 h (B). NO production was calculated in culture media by Griess reagent (B). Each bar represents means ± S.D. of three independent experiments. ∗p <0.05 compared to the group treated with LPS. |
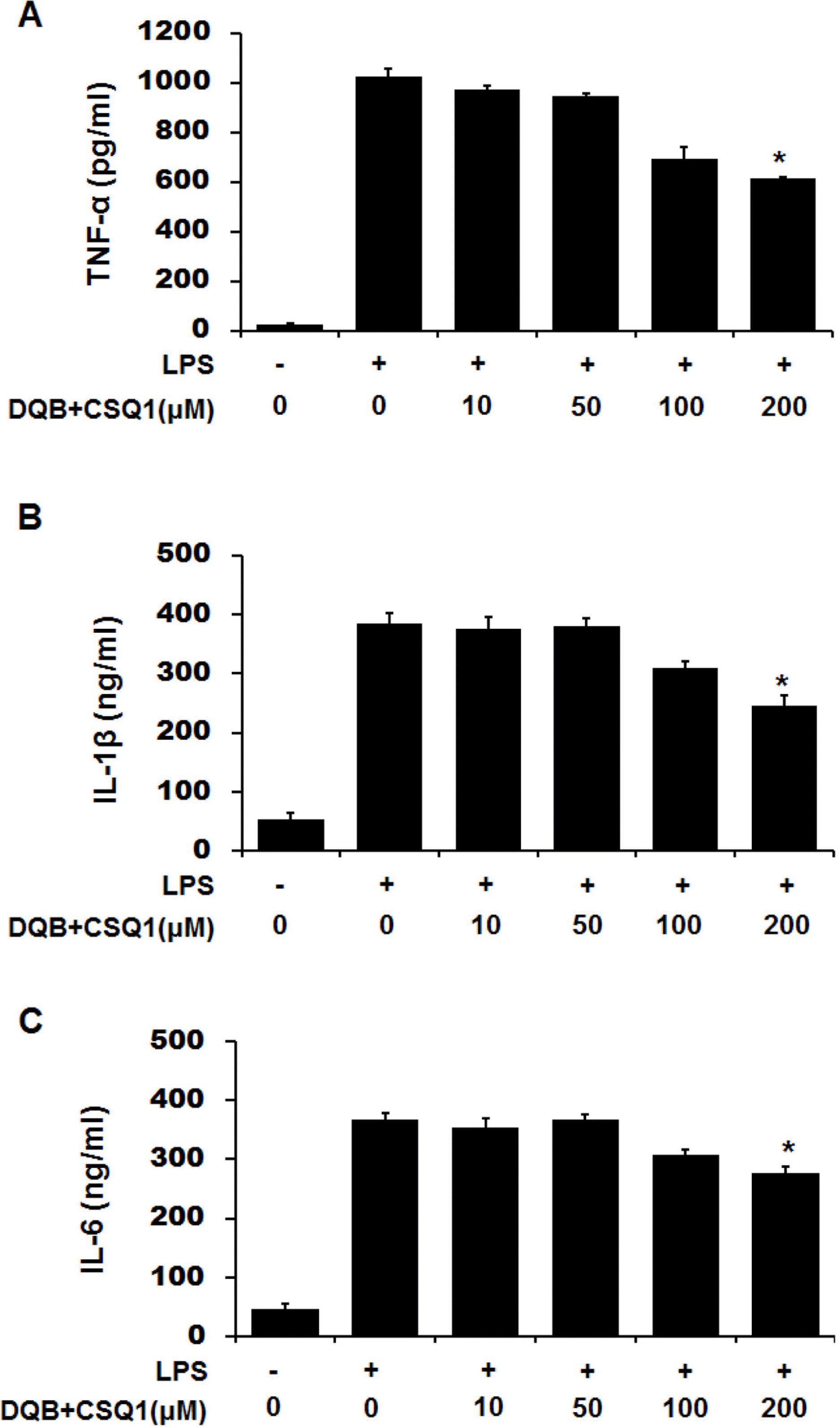 | Fig. 3.Effect of DQB and CSQ1 mixture on LPS-induced proinflammatory cytokines in RAW 264.7 cells. Cells were treated with various concentrations of DQB and CSQ1 mixture for 1 h and incubated with LPS during 24 h. Productions of TNF-α (A), IL-1β (B), and IL-6 (C) were measured in culture media by ELISA. Each bar represents means ± S.D. of three independent experiments. ∗p < 0.05 compared to the group treated with LPS. |
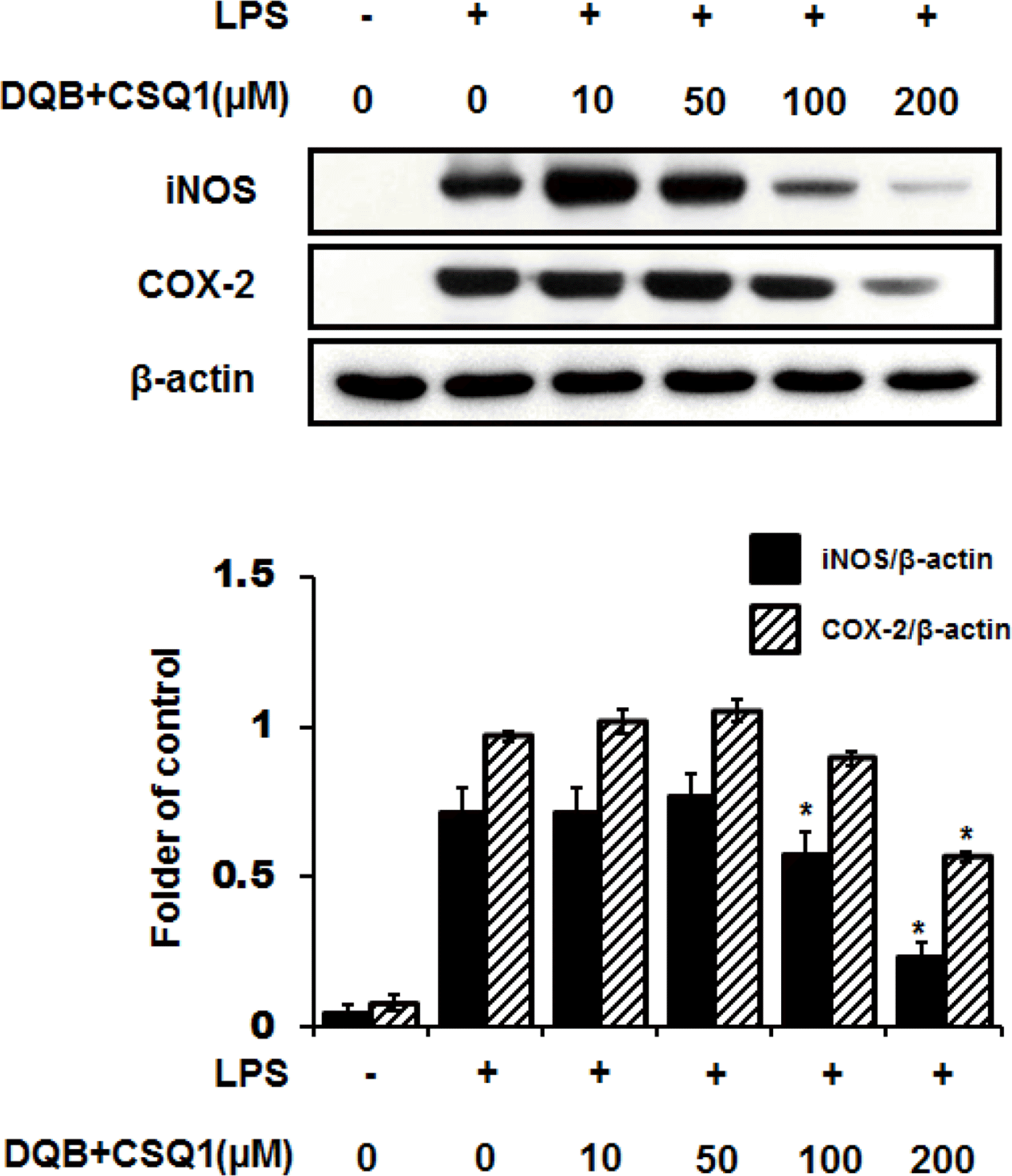 | Fig. 4.Effect of DQB and CSQ1 mixture on LPS-induced iNOS and COX-2 expression in RAW 264.7 cells. Cells were treated with various concentrations of DQB and CSQ1 mixture for 1 h and incubated with LPS during 24 h. The expression levels of iNOS and COX-2 in cells were analyzed by Western blot. Each bar represents means ± S.D. of three independent experiments. ∗p < 0.05 compared to the group treated with LPS. |
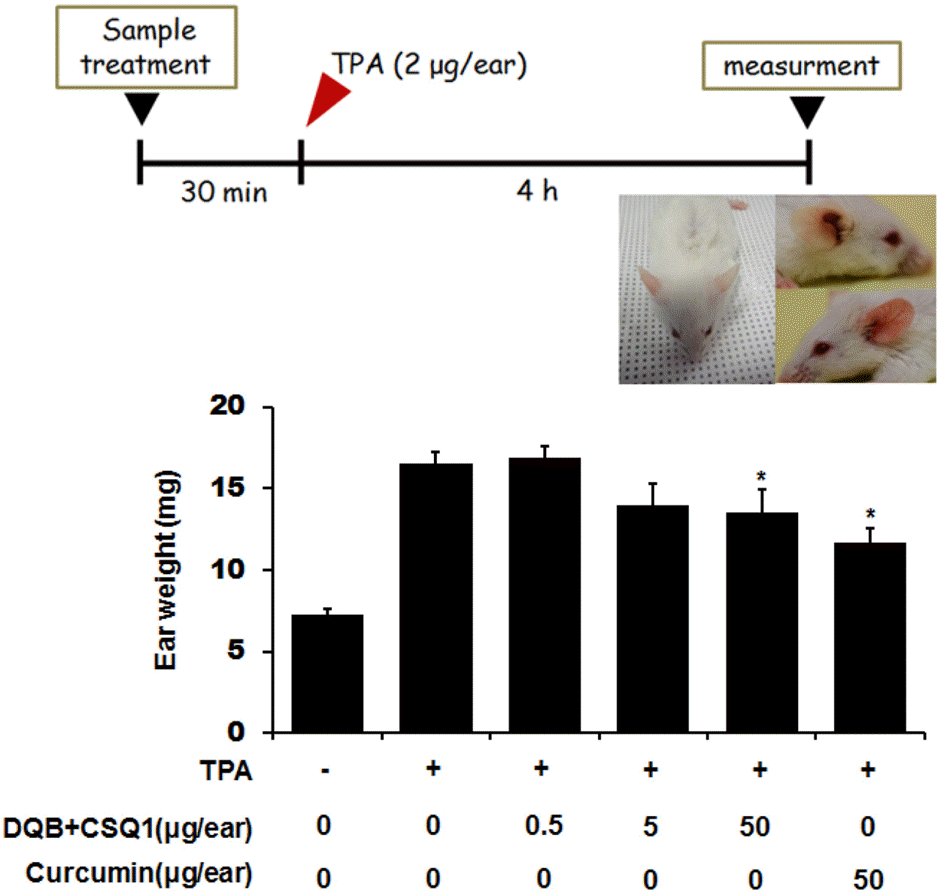 | Fig. 5.Effect of DQB and CSQ1 mixture on TPA-induced ear edema in ICR mouse. Mice were administrated with or without the various concentrations of DQB and CSQ1 mixture. Curcumin was used as a positive control. TPA (2 μg/ear) was dropped onto the surface of the ear. The mice were sacrificed 4 h after the application of TPA. An ear disc 9.0 mm diameter was punched out of each ear and weighted. Values are shown as means ± S.D. of n=5 mice. ∗ p < 0.05 compared to the group treated with TPA. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download