Abstract
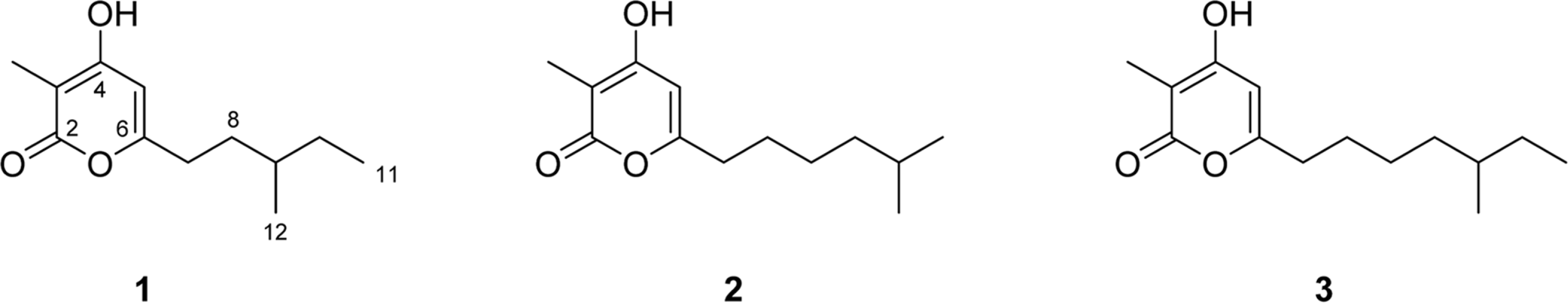
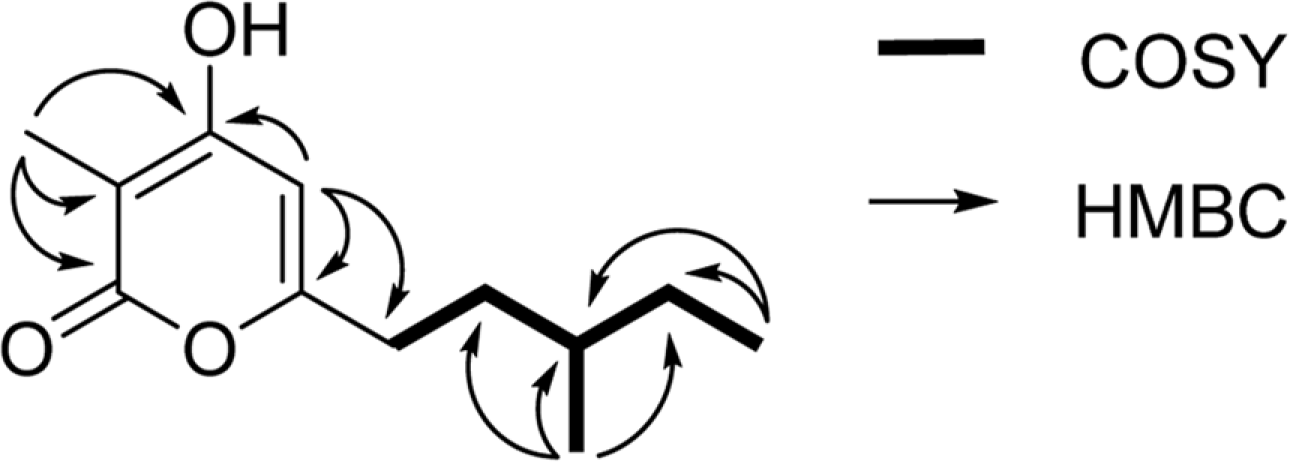
A new α-pyrone derivative, violapyrone J (1), and along with the two known violapyrones B (2) and C (3) were isolated from the fermentation broth of a marine actinomycete Streptomyces sp. SC0718. The structure of violapyrone J (1) was elucidated from 1D and 2D NMR spectroscopic analyses.
REFERENCES
(1). Bérdy J. J.Antibiot. 2005; 58:1–26.
(2). Buchanan G. O., Williams P. G., Feling R. H., Kauffman C. A., Jensen P. R., Fenical W.Org. Lett. 2005; 7:2731–2734.
(3). Boonlarppradab C., Kauffman C. A., Jensen P. R., Fenical W.Org. Lett. 2008; 10:5505–5508.
(4). Sato S., Iwata F., Mukai T., Yamada S., Takeo J., Abe A., Kawahara H. J.Org. Chem. 2009; 74:5502–5509.
(5). Pérez M., Crespo C., Schleissner C., Rodríguez P., Zúñiga P., Reyes F. J.Nat. Prod. 2009; 72:2192–2194.
(6). Asolkar R. N., Freel K. C., Jensen P. R., Fenical W., Kondratyuk T. P., Park E. J., Pezzuto J. M. J.Nat. Prod. 2009; 72:396–402.
(7). McArthur K. A., Mitchell S. S., Tsueng G., Rheingold A., White D. J., Grodberg J., Lam K. S., Potts B. C. J.Nat. Prod. 2008; 71:1732–1337.
(8). Mehbub M. F., Lei J., Franco C., Zhang W.Mar. Drugs. 2014; 12:4539–4577.
(9). Takami H., Inoue A., Fuji F., Horikoshi K.FEMS Microbiol. Lett. 1997; 152:279–285.
(10). Cutignano A., Fontana A., Renzulli L., Cimino G. J.Nat. Prod. 2003; 66:1399–1401.
(11). Fu P., Liu P., Qu H., Wang Y., Chen D., Wang H., Li J., Zhu W. M. J.Nat. Prod. 2011; 74:2219–2223.
(12). Zhang J., Jiang Y., Cao Y., Liu J., Zheng D., Chen X., Han L., Jiang C., Huang X. J.Nat. Prod. 2013; 76:2126–2130.
(13). Dictionary of Natural Products on DVD: version 21.2. Chapman & Hall/CRC;London: 2013.
(14). Li C., Nitka M. V., Gloer J. B., Campbell J., Shearer C. A. J.Nat. Prod. 2003; 66:1302–1306.
(15). Ohno H., Saheki T., Awaya J., Nakagawa A., Omura S. J.Antibiot. 1978; 31:1116–1123.
Table 1.
NMR data of violapyrone J (1) in CD3 OD. (δ in ppm)a
| No. | 1 | |||
|---|---|---|---|---|
| δC, mult.b | δH (J in Hz) | COSY | HMBC | |
| 2 | 181.1, C | |||
| 3 | 96.6, C | |||
| 4 | 171.4, C | |||
| 5 | 108.2, CH | 5.77, s | 3, 6, 7 | |
| 6 | 163.4, C | |||
| 7 | 32.3, CH2 | 2.35-2.45, m | 8 | 5 |
| 8 | 35.3, CH2 | 1.70, 1.20, m | 7, 9 | |
| 9 | 35.4, CH | 1.45, m | 8, 10,12 | |
| 10 | 30.6 CH2 | 1.40, 1.20, m | 9, 11 | 11, 12 |
| 11 | 12.0, CH3 | 0.91, t (7.0) | 10 | |
| 12 | 19.6, CH3 | 0.93 d (6.3) | 9 | 8 |
| 3-Me | 9.0, CH3 | 1.81, s | 2, 3, 4 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download