Abstract
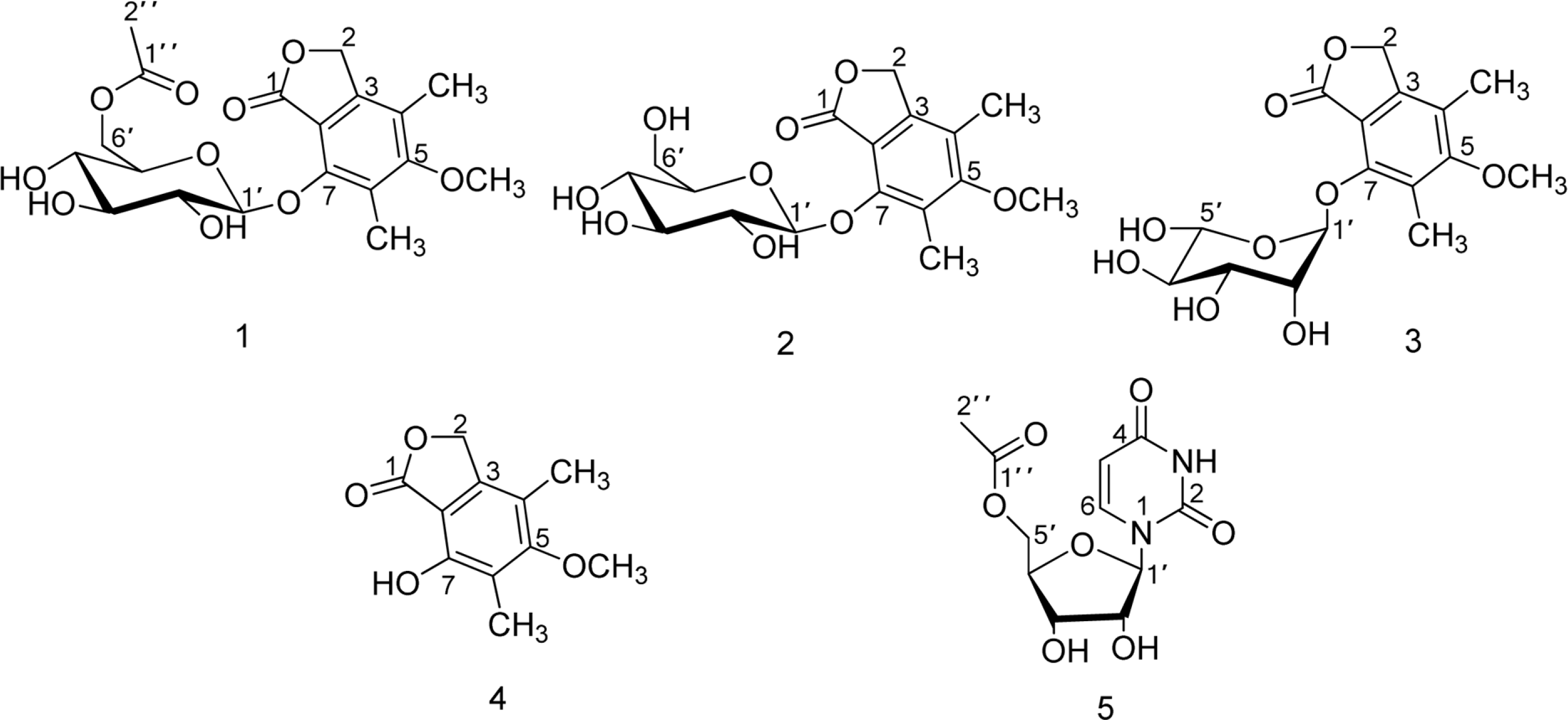
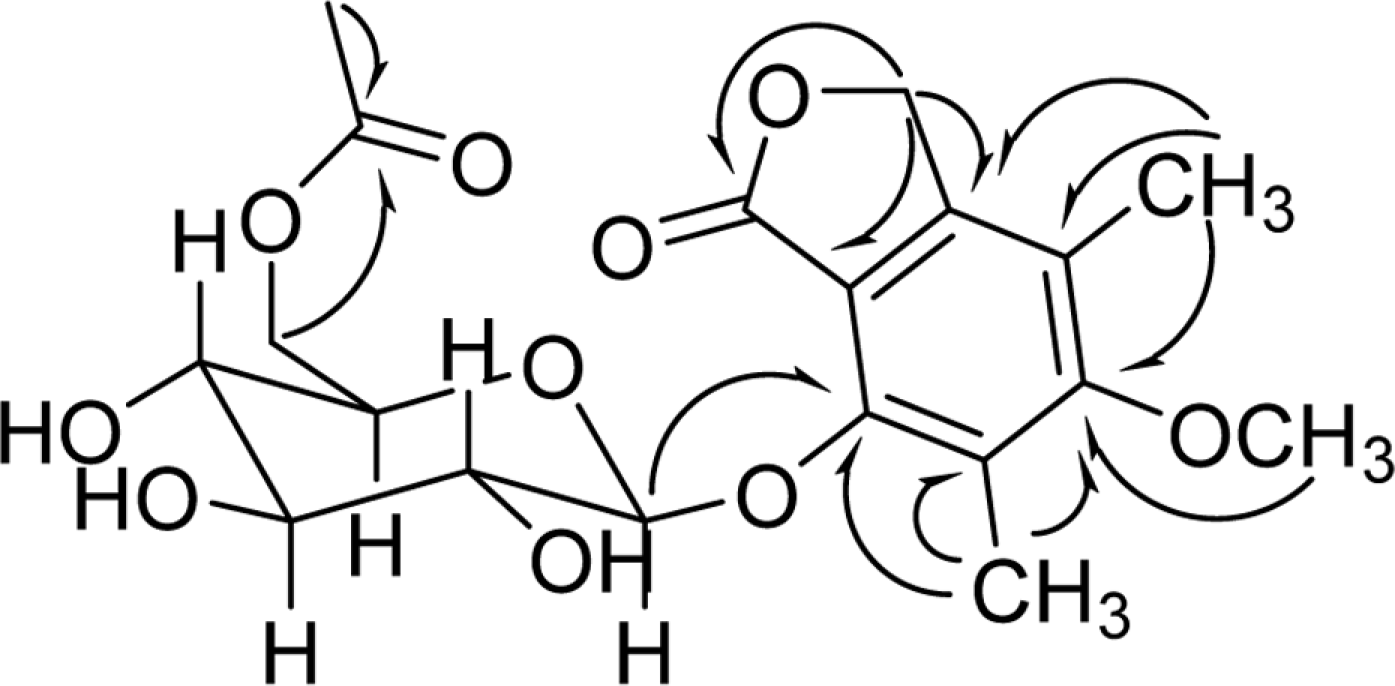
Chemical investigation of the fermentation broth of a Soft Coral-Derived fungus Pestalotiopsis sp., led to the isolation of a new phthalide derivative, pestalotiolide A (1), three known analogues (2, 3 and 4), along with 5'-O-acetyl uridine (5) first isolated as a natural product. The structure of the new compound (1) was established by comprehensive spectroscopic analysis and chemical methods. Compounds 1 - 4 possessed varying degrees of antiviral activities, which was reported for the first time. Compared to the positive control ribavirin (IC50 = 418.0 µM), pestalotiolide A (1) exhibited significant anti-EV71 activity in vitro, with an IC50 value of 27.7µ M. Furthermore, the preliminary structure-activity relationship of antiviral activities was also discussed.
Go to : 
REFERENCES
(1). Yang X. L., Zhang J. Z., Luo D. Q.Nat. Prod. Rep. 2012; 29:622–641.
(2). Xu J., Ebada S. S., Proksch P.Fungal. Divers. 2010; 44:15–31.
(3). Li J., Li L., Si Y., Jiang X., Guo L., Che Y.Org. Lett. 2011; 13:2670–2673.
(4). Klaiklay S., Rukachaisirikul V., Tadpetch K., Sukpondma Y., Phongpaichit S., Buatong J., Sakayaroj J.Tetrahedron. 2012; 68:2299–2305.
(5). Beekman A. M., Barrow R. A. J.Nat. Prod. 2013; 76:2054–2059.
(6). Wu B., Wu X., Sun M., Li M.Mar. Drugs. 2013; 11:2713–2721.
(7). Wang J., Wei X., Lu X., Xu F., Wan J., Lin X., Zhou X., Liao S., Yang B., Tu Z., Liu Y.Tetrahedron. 2014; 70:9695–9701.
(8). Arunpanichlert J., Rukachaisirikul V., Phongpaichit S., Supaphon O., Sakayaroj J.Tetrahedron. 2015; 71:882–888.
(9). Wang J., Wei X., Qin X., Chen H., Lin X., Zhang T., Yang X., Liao S., Yang B., Liu J., Zhou X., Tu Z., Liu Y.Phytochem. Lett. 2015; 12:59–62.
(10). Wei M. Y., Li D., Shao C. L., Deng D. S., Wang C. Y.Mar. Drugs. 2013; 11:1050–1060.
(11). Chen G., Tian L., Wu H. H., Bai J., Lu X., Xu Y., Pei Y. H. J.Asian Nat. Prod. Res. 2012; 14:759–763.
(12). Simeó Y., Sinisterra J. V., Alcántara A. R.Green Chem. 2009; 11:855–862.
(13). Schneider G., Anke H., Sterner O.Nat. Prod. Lett. 1997; 10:133–138.
(14). Xing J. G., Deng H. Y., Luo D. Q. J.Asian Nat. Prod. Res. 2011; 13:1069–1073.
(15). Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H.Virol. J. 2008; 5:107.
Go to : 
Table 1.
1H and13C NMR data of 1 in CD3 OD. (δ in ppm, J in Hz, 500 MHz for 1H and 125 MHz for 13C)
Table 2.
Antiviral activities of the tested compounds 1 - 5a




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download