Abstract
Nasotracheal intubation is generally a useful maxillofacial surgery that provides good surgical access for intraoral procedures. When nasotracheal intubation is difficult, laryngeal mask airway (LMA) insertion can be performed, and the flexible LMA™ (FLMA) is also useful for anesthetic management. However, the FLMA provides limited access to the mouth, which restricts the insertion of instrumentation and confines the surgical field available. Here, we present our experience using the FLMA airway management for dental treatment cases involving difficulty with intubation.
Nasotracheal intubation is commonly used for maxillofacial surgery [123], particularly intraoral procedures [23], as it provides unrestricted access to the mouth and enlarges the surgical field, facilitating the insertion of instrumentation. However, the incidence of unanticipated difficulty with nasotracheal intubation, although low, is consistent. Devices such as the laryngeal mask airway (LMA) and fiberoptic scope are effective in establishing an airway during a difficult intubation [234], and the LMA can be used for maxillofacial surgery [56]. Of note, the flexible LMATM (FLMA; The LMA Company, San Diego CA, USA) has a long cuff portion and non-rigid flexometallic tube [4], which might decrease the risk of developing an airway disorder. Here, we present our experience using FLMA airway management for two dental treatment cases involving difficulty with intubation.
A 7-year-old boy, 107 cm in height and 17 kg in weight, had been previously presented with fever and convulsion and was diagnosed with acute encephalopathy at the age of 3 years. Dental treatment at our hospital was scheduled under general anesthesia due to his multiple caries and uncooperative behavior.
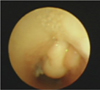
At 1 month after his initial diagnosis, the patient had received a tracheotomy for chronic respiratory failure, and at 5 years of age, he received trachea closure surgery because his respiratory condition stabilized. After computed tomography (CT) confirmation of tracheal granulation tissue under the vocal cords at 7 years of age, the patient regularly received follow-up observations (Fig. 1). The neurological status of the patient was poor with severe mental retardation and cerebral paralysis. He could not speak or walk without support and we could not communicate with him. The patient had been taking antiepileptic drugs daily and was without seizure activity for more than two years. However, due to tracheal granulation, the patient had a few episodes of wheezing and stridor. On admission, clinical assessment of his airway was difficult, but his respiratory condition was stable and his oxygen saturation (SpO2) was 98% in room air. No abnormal findings were observed on a chest X-ray, in laboratory data, or in cardiac function.
On the day of dental treatment, no premedication was administered, and the patient was transferred to the operating room. Anesthesia was induced with inhalation of sevoflurane at 1–8% in oxygen after the initiation of noninvasive monitoring for SpO2 (100%). After the loss of consciousness, mask ventilation with an oral airway was uncomplicated, and we began electrocardiogram (ECG; sinus rhythm), blood pressure (BP; 90/58 mmHg), and heart rate (HR; 105 bpm) monitoring. Atropine at 100 mcg and rocuronium at 6 mg were administered after peripheral intravenous access was obtained. Insertion was carried out without difficulty with a size 2 FLMA (Fig. 2). Anesthesia was maintained with sevoflurane at 1–3% in air and oxygen. Blood pressure was maintained at 85–110/38–68 mmHg and HR was 80–100 bpm with acetaminophen. In the surgical field, a rubber dam isolation technique was used to prevent contamination due to saliva or hemorrhage. The conservative treatment and tooth extraction were completed in 135 min without any surgical or anesthetic problems. Blood loss was minimal during the operation and the patient received a total of 436 mL of lactated Ringer's solution with 1% glucose. Urine volume was 60 mL. The respiratory condition was stable after removing the FLMA. The patient left the hospital one day after the dental surgery, and no remarkable changes or complications have occurred thereafter.
The patient was a 2-year-old girl, 82.9 cm in height and 9.7 kg in weight, who had been diagnosed with right hypoplasia, pulmonary artery sling (PAS), and ventricular septal disease (VSD) at birth. Her mother found multiple caries in association with an eating disorder. The patient was transferred to our hospital after relocation of the left PA and VSD closure. She had a history of difficult intubation (tube could not be forwarded); therefore, we scheduled her dental treatment under general anesthesia.
The patient had no abnormal findings on preoperative laboratory test. The assessment of her airway was difficult due to her uncooperative behavior. A preoperative CT showed a tracheal deviation to the right and right hypoplasia. Upon physical examination, intermittent stridor was detected using chest auscultation. In addition, her respiratory condition was stable and SpO2 was 98%. The patient receives treatment daily with a short-acting beta-agonist and an inhaled corticosteroid. Her last respiratory attack had occurred approximately 1 year before our assessment.
On the day of dental treatment, no premedication was administered, and the patient was transferred to the operating room. Anesthesia was induced with inhalation of sevoflurane at 1–8% in oxygen after the start of noninvasive monitoring for SpO2 (98%). After the loss of consciousness, mask ventilation with an oral airway was uncomplicated and monitoring of ECG (sinus rhythm), BP (102/64 mmHg), and HR (161 bpm) began. Fentanyl at 20 mcg, atropine at 50 mcg, and rocuronium at 6 mg were administered after peripheral intravenous access was obtained. Subsequently, we placed a size 2 FLMA. Anesthesia was maintained with sevoflurane at 1–3% in air and oxygen. BP was maintained at 78–100/40–62 mmHg, HR was 125–140 bpm, and end-tidal CO2 (EtCO2) was 35–40 mmHg. In the surgical field, the rubber dam isolation technique was used. The conservative treatment was completed in 138 min without any surgical or anesthetic problems. There was minimal blood loss during the operation and the patient received a total of 118 L of lactated Ringer's solution with 1% glucose. Urine volume was 40 mL. After removing the FLMA, her respiratory condition was stable. The patient left the hospital 1 day after the treatment, and no remarkable changes or complications have been observed since.
In our experience, FLMA could be used to obtain an airway during general anesthesia. The FLMA passed beyond the tongue, forming a laryngeal seal, eliminating the risk of causing upper airway obstruction [456]. The cuff portion of the FLMA is similar to that of the classic version of the LMA but the FLMA has a long and non-rigid flexometallic tube [78]. This design allows for more movement of the tube without displacement of the cuff or a loss of the seal. The FLMA has been used for dental treatment and for head and neck surgery [8].
Airway injury after a tracheotomy can be a serious clinical problem [910]. Tracheotomy tubes can cause severe stomal stenosis in the trachea. The incidence of tracheal stenosis following tracheotomy ranges from 0.6-6% [9]. Tracheal stenosis mostly occurs at the cuff in the tube. The most common complication is the formation of tracheal granulations at the suture line [9]. A frequent complication is the development of granulation tissue, which can cause airway complication or result in airway stenosis. Granulation tissue at the stoma and the trachea has been described as a late complication, resulting in bleeding, drainage, and airway problems [910]. Obstruction that is related to granulation tissue has been cited as the cause of death in several patients. The incidence of granulation tissue is 0.3% to 80% [10]. In terms of airway access, tracheal intubation may be better than LMA insertion. However, in the present case, we avoided intubation to prevent airway obstruction due to tracheal tube-induced bleeding from the granulation tissue.
PAS is an extremely rare congenital heart anomaly [111213] that occurs when the left pulmonary artery arises from the right pulmonary artery while forming a sling around the trachea, causing tracheal compression [12]. It frequently accompanies a varying degree of tracheal stenosis. PAS correction can be used to treat respiratory symptoms and/or signs that are the result of tracheal stenosis. In case 2, the patient was previously diagnosed with PAS and previously had a difficult intubation under anesthesia. Forced insertion of the tracheal tube or repeated attempts might damage the trachea. Therefore, we did not attempt to perform intubation but selected to insert the FLMA.
A limitation with the use of the FLMA is the inability to isolate the airway and to protect against the risk of aspiration; this limitation is particularly important when the surgical site within the oral cavity is near the pharynx, as an FLMA procedure in the oral cavity under this circumstance might increase the risk of airway obstruction [5].
In conclusion, a general use of the FLMA during dental treatment under general anesthesia might be effective for the management of a difficult airway due to conditions such as tracheal granulation or rare congenital anomalies.
Figures and Tables
References
1. Prasanna D, Bhat S. Nasotracheal Intubation: An Overview. J Maxillofac Oral Surg. 2014; 13:366–372.

2. Monclus E, Garcés A, Artés D, Mabrock M. Oral to nasal tube exchange under fibroscopic view: a new technique for nasal intubation in a predicted difficult airway. Paediatr Anaesth. 2008; 18:663–666.

3. Arslan Zİ, Ozdal P, Ozdamar D, Agır H, Solak M. Nasotracheal intubation of a patient with restricted mouth opening using a McGrath MAC X-Blade and Magill forceps. J Anesth. 2016; 30:904–906.

4. Arisaka H, Matsumoto M, Furuya M, Sakuraba S, Yoshida K. Application of nasal flexible laryngeal mask airway in anesthesia for oral surgery. J Anesth. 2007; 21:99–101.

5. Bennett J, Petito A, Zandsberg S. Use of the laryngeal mask airway in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1996; 54:1346–1351.

6. Choo CY, Koay CK, Yoong CS. A randomised controlled trial comparing two insertion techniques for the Laryngeal Mask Airway Flexible™ in patients undergoing dental surgery. Anaesthesia. 2012; 67:986–990.

7. Brimacombe J, Berry A. The laryngeal mask airway for dental surgery--a review. Aust Dent J. 1995; 40:10–14.

8. Asahi Y, Fujii R, Usui N, Kagamiuchi H, Omichi S, Kotani J. Anesthetic management by laryngeal mask airway in a patient with a history of difficult intubation resulting in dental injuries. Anesth Prog. 2015; 62:20–21.

9. Sarper A, Ayten A, Eser I, Ozbudak O, Demircan A. Tracheal stenosis aftertracheostomy or intubation: review with special regard to cause and management. Tex Heart Inst J. 2005; 32:154–158.
10. Yaremchuk K. Regular tracheostomy tube changes to prevent formation of granulation tissue. The Laryngoscope. 2003; 113:1–10.

11. Kosucu M, Eroglu A, Besir A, Cansu A. Using Proseal LMA and I-gel for difficult airway management in patient with diffuse tracheal stenosis and pulmonary artery sling. Bratisl Lek Listy. 2013; 114:418–420.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download