Abstract
Digital dentistry has influenced many dental procedures, such as three-dimensional (3D) diagnosis and treatment planning, surgical splints, and prosthetic treatments. Patient-specific protective appliances (PSPAs) prevent dental injury during endotracheal intubation. However, the required laboratory work takes time, and there is the possibility of tooth extraction while obtaining the dental impression. In this technical report, we utilized new digital technology for creating PSPAs, using direct intraoral scanners and 3D printers for dental cast fabrication.
Dental injuries that occur during endotracheal intubation are serious complications of general anesthesia, with an incidence of 0.17 to 12.1% [1234]. These injuries may occur due to misuse of the laryngoscope; however, pre-existing dental risk factors, such as periodontal disease, weakened coronal structure, and history of dental implant treatment, are the most common causes [2]. Prevention of such injuries is important in order to avoid medico-legal issues. Several methods are used to prevent dental injury during endotracheal intubation, such as the use of adhesive plaster, gauge roll, folded tapes, transformed intubation blades, and preformed tooth protectors [35]. The most effective approach for prevention is a pre-anesthetic dental consultation and fabrication of a patient-specific protective appliance (PSPA).
However, a pre-anesthetic dental consultation is not always possible, since surgery is sometimes performed on an urgent or emergency basis. Other reasons for avoiding the dental consultation include the stress of the procedure for the patient and medical doctor, as well as the time and cost involved.
If indicated during consultation, a PSPA is created using the following procedure: 1) a tooth impression is made using an irreversible hydrocolloid impression material, 2) a dental cast is fabricated with improved stone pouring and trimming, 3) the splint is fabricated with a vacuum former, and 4) the splint is trimmed and delivered [6]. These steps are time-consuming, not only for the patient, but for the dentist or dental laboratory worker as well [789].
Recently, digital dentistry has become a popular topic worldwide. Using digital technology, we can obtain three-dimensional (3D) images of tooth structure using intraoral scanners, cast scanners, and impression scanners. Furthermore, we can create 3D prints of prosthetic inlays or crowns for oral rehabilitation. For accurate placement of a dental implant, 3D digital diagnosis, treatment planning, and computer-guided surgical splints can be used during surgery. Digital dentistry is not perfect; however, it can save time and reduce the amount of dental material used and wasted.
We used dentiforms (Standard dentiform ANKA-4, Frasaco, Tettnang, Germany) for our evaluation of the digital PSPAs. For comparison, PSPAs were also fabricated using the following conventional method. First, an impression with an irreversible hydrocolloid material (Cavex CA37, CAVEX Holland BV, Haarlem, The Netherlands) was taken. After the irreversible hydrocolloid was set, improved dental stone (SNOW ROCK, DK MUNGYO, Gyeongnam, Korea) was poured carefully to avoid forming voids. The dental cast was fabricated by trimming the excessive stone material. The PSPA was then made using a vacuum former (Biostar, SCHEU-DENTAL, Iserlohn, Germany) with a soft silicon film (Bioplast, SCHEU-DENTAL, Iserlohn, Germany). For the digital PSPAs, intraoral scanning was performed with a scanner (CS3500, Carestream Dental, Georgia, USA), and the stereolithography (STL) files obtained were transferred to 3D computer-assisted design (CAD) software (Exocad, Darmstadt, Germany). A stereolithography apparatus (SLA) printer (Zenith, Dentis, Korea) with an acyl acrylate oligomer based photopolymer resin (ZMD-1000B, Dentis, Korea) was used for the 3D printing of the dental cast. The scanned STL files were designed by Exocad software (Fig. 1).
After acquisition of the 3D printed dental cast, the same procedure was followed for the PSPA using the vacuum former. The margin of the silicon film was trimmed to within 1 mm of the subgingival area (Fig. 2).
We then tested the accuracy and acceptance of the conventional PSPAs and the digital PSPAs. We tested both arches (maxilla and mandible). The digital process took less time for the intraoral scanning and more time for the 3D printing process than the conventional method. Improved work flow was observed with digital dentistry, as it takes less time for the intraoral scanning [12], does not waste materials, and eliminates the risk of accidental tooth extraction while obtaining the impressions. Mouth guards made with the digital method fit well and had similar accuracy to those made with the conventional method (Fig. 3). They also may be easier for dentists and anesthesiologists to handle.
Technical innovations enable our society to move forward in the digital world, and dentistry is influenced by such technical developments. Digital dentistry affects our routine dental procedures, such as simple prosthetic treatments, implant treatment planning, and 3D printed models. The use of digital dentistry can save time for patients, as well as doctors, and reduce the amount of dental materials used [13].
Creating PSPAs is not difficult and does not require precise laboratory procedures compared to those of other prosthetics; however, their creation takes a lot of time. At least 2 h are required to make a PSPA for one arch [14]. Furthermore, for patients with severe periodontitis and hypermobile teeth, tooth extraction can occur while obtaining conventional irreversible hydrocolloid impressions. We utilized a direct intraoral scanning procedure in order to reduce these complications. Intraoral scanning for one maxillary or mandibular arch takes only 20 min. Once the scan is complete, sharing and transferring the STL file allows for 3D printing, saving time and preventing the risk of tooth extraction. We used an SLA printer manufactured by Dentis that had demonstrated similar accuracy to that of conventional methods with no distortion. Therefore, we thought this device was sufficient for the dentiform test.
With an STL file, any 3D printer can print digital dental casts with ease. There are several types of 3D printers available, such as FDM (Fused Deposition Modeling), DLP (Digital Light Processing), and SLA [13]. The cost, accuracy, and materials used vary with each type of 3D printer. The FDM printer is the most widely used 3D printer, so the cost of the machine and the filament are economical; however, the accuracy is relatively low.
The DLP printer uses a projector to set the resin, and the accuracy and surface characteristics are very good. However, the cost of the device and resin are high. The SLA printer is a high-end 3D printer, so the accuracy is perfect for dental prostheses. However, the cost of the printer and resin are also high. The materials used also influence the quality of the 3D dental casts. Many materials are used for 3D printing, such as PLA (Polylactic Acid), ABS (Acrylonitrile Butadiene Styrene), PMMA (Polymethyl methacrylate), and PVA (Polyvinyl alcohol).
We used an SLA printer with an acyl acrylate oligomer based photopolymer resin. Financially, the FDM is the most promising printer for creating 3D dental casts; however, the temperature required for the vacuum former was too high to be used repeatedly.
3D printing can be used to make a PSPA after determining the optimal design of the splint using CAD software. With recent technology, it is possible to design and print the splint-shaped product; however, there are no regulations concerning the safety of the 3D printing materials used for intraoral appliances in Korea. Therefore, splint fabrication with 3D printing technology is possible, yet we cannot use the splint for the patient, since the safety has not been determined. If regulations related to 3D printing materials are established, we can then attempt a full-digital sequential procedure for creating a PSPA, with intraoral scanning, CAD, and direct 3D printing of the splint.
In this technical note, we suggested methods for PSPA fabrication using digital intraoral scanning and 3D printing of a dental cast. Further research should be conducted concerning the clinical use of 3D printing, such as 1) the optimal protocol for 3D printed dental casts, based on the 3D printing material (PLA, ABS, and PMMA), 2) comparison of the efficacy of printing dental casts depending on the type of 3D printer (SLA type, DLP type, and FDM type), and 3) the stable splint design and thickness required for each 3D printing material to withstand manipulation by anesthesiologists.
Figures and Tables
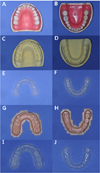 | Fig. 2Models made using the different methods (A, B-Original dentiform models; C, D-Gypsum models with alginate impression; E, F-PSPAs made using the Gypsum models; G, H-3D printing models; I, J-PSPAs made using the 3D printing models). PSPA: patient specific protective appliance, 3D: three-dimensional. |
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0822).
References
1. Chen JJ, Susetio L, Chao CC. Oral complications associated with endotracheal general anesthesia. Ma Zui Xue Za Zhi. 1990; 28:163–169.
2. Lockhart PB, Feldbau EV, Gabel RA, Connolly SF, Silversin JB. Dental complications during and after tracheal intubation. J Am Dent Assoc. 1986; 112:480–483.

3. Burton JF, Baker AB. Dental damage during anaesthesia and surgery. Anaesth Intensive Care. 1987; 15:262–268.

4. Deppe H, Reeker W, Horch HH, Kochs E. Tooth injury during intubation--diagnostic and therapeutic aspects. Anasthesiol Intensivmed Notfallmed Schmerzther. 1998; 33:722–725.
5. Ghabash MB, Matta MS, Mehanna CB. Prevention of dental trauma during endotracheal intubation. Anesth Analg. 1997; 84:230–231.

6. Lee KH, You TM, Park W, Lee SH, Jung BY, Pang NS, et al. Protective dental splint for oroendotracheal intubation: Experience of 202 cases. J Dent Anesth Pain Med. 2015; 15:17–23.

7. Akyalcin S, Cozad BE, English JD, Colville CD, Laman S. Diagnostic accuracy of impression-free digital models. Am J Orthod Dentofacial Orthop. 2013; 144:916–922.

8. Nedelcu RG, Persson AS. Scanning accuracy and precision in 4 intraoral scanners: An in vitro comparison based on 3-dimensional analysis. J Prosthet Dent. 2014; 112:1461–1471.

9. De Luca Canto G, Pacheco-Pereira C, Lagravere MO, Flores-Mir C, Major PW. Intra-arch dimensional measurement validity of laser-scanned digital dental models compared with the original plaster models: A systematic review. Orthod Craniofac Res. 2015; 18:65–76.

10. Renne W, Ludlow M, Fryml J, Schurch Z, Mennito A, Kessler R, et al. Evaluation of the accuracy of 7 digital scanners: An in vitro analysis based on 3-dimensional comparisons. J Prosthet Dent. 2016.
11. Mangano F, Shibli JA, Fortin T. Digital dentistry: New materials and techniques. Int J Dent. 2016; 2016:5261247.

12. Patzelt SB, Lamprinos C, Stampf S, Att W. The time efficiency of intraoral scanners: An in vitro comparative study. J Am Dent Assoc. 2014; 145:542–551.
14. Kloeg EF, Collys K. Materials for mouth protectors. Rev Belge Med Dent (1984). 2003; 58:21–33.
15. Manka-Malara K, Gawlak D, Hovhannisyan A, Klikowska M, Kostrzewa-Janicka J. Dental trauma prevention during endotracheal intubation--review of literature. Anaesthesiol Intensive Ther. 2015; 47:425–429.
16. Monaca E, Fock N, Doehn M, Wappler F. The effectiveness of preformed tooth protectors during endotracheal intubation: An upper jaw model. Anesth Analg. 2007; 105:1326–1332.

17. Sato N, Shingu K. Another reason to choose the left molar approach of laryngoscopy: To spare the incisor teeth. Anesthesiology. 2002; 96:1279.

19. Hagelsten JO, Marvitz L. Prevention of dental damage during anesthesia. Z Prakt Anasth. 1971; 6:195–205.
21. Newland MC, Ellis SJ, Peters KR, Simonson JA, Durham TM, Ullrich FA, et al. Dental injury associated with anesthesia: A report of 161,687 anesthetics given over 14 years. J Clin Anesth. 2007; 19:339–345.

22. Cass NM. Medicolegal claims against anaesthetists: A 20 year study. Anaesth Intensive Care. 2004; 32:47–58.

23. Owen H, Waddell-Smith I. Dental trauma associated with anaesthesia. Anaesth Intensive Care. 2000; 28:133–145.

24. Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016; 12:1–222.
25. Warner ME, Benenfeld SM, Warner MA, Schroeder DR, Maxson PM. Perianesthetic dental injuries: Frequency, outcomes, and risk factors. Anesthesiology. 1999; 90:1302–1305.

26. Magnin C, Bory EN, Motin J. Tooth injuries during intubation: A new preventive device. Ann Fr Anesth Reanim. 1991; 10:171–174.
27. Folwaczny M, Hickel R. Oro-dental injuries during intubation anesthesia. Anaesthesist. 1998; 47:707–731.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download