This article has been
cited by other articles in ScienceCentral.
Abstract
Intraoperative airway obstruction is perplexing to anesthesiologists because the patient may fall into danger rapidly. A 74-year-old woman underwent an emergency incision and drainage for a deep neck infection of dental origin. She was orally intubated with a 6. 0 mm internal diameter reinforced endotracheal tube by video laryngoscope using volatile induction and maintenance anesthesia (VIMA) with sevoflurane, fentanyl (100 µg), and succinylcholine (75 mg). During surgery, peak inspiratory pressure increased from 22 to 38 cmH2O and plateau pressure increased from 20 to 28 cmH2O. We maintained anesthesia because we were unable to access the airway, which was covered with surgical drapes, and tidal volume was delivered. At the end of surgery, we found a longitudinal fold inside the tube with a fiberoptic bronchoscope. The patient was reintubated with another tube and ventilation immediately improved. We recognized that the tube was obstructed due to dissection of the inner layer.
Keywords: Intubation, Bronchoscopes, Ludwig's Angina, Reinforced Endotracheal Tube
Endotracheal intubation is performed routinely by anesthesiologists. The establishment of the endotracheal tube during both elective and emergency situations has allowed for immediate life-saving interventions during resuscitation, maintenance of oxygenation and ventilation, and delivery of inhaled anesthesia. However, the obstruction of an endotracheal tube is a potentially life-threatening complication [
1]. Herein we report a case of obstruction of a reinforced endotracheal tube due to dissection of the inner layer in a patient who was difficult to intubate and was scheduled for an incision and drainage of a deep neck infection that was of dental origin.
CASE REPORT
A 74-year-old woman (weight = 48 kg; height = 151 cm) presented to the emergency room with complaints of pain on sublingual and tongue swelling. Neck computerized tomography confirmed a deep neck infection, and she was diagnosed with Ludwig's angina. She was scheduled for emergency incision and drainage. Preoperative evaluation revealed that she had hypertension and bronchiectasis. An electrocardiogram (ECG) was performed and yielded no abnormal findings. Chest radiograph showed underlying bronchiectasis in both lungs. Results from laboratory tests showed an increased white blood cell count. An 18-gauge intravenous catheter was inserted into the right arm and lactated Ringer's solution was instilled. She entered the operating room without premedication. Oxygen saturation (SpO2), non-invasive blood pressure (NIBP), and ECG were monitored. The patient's initial vital signs were as follows: NIBP = 120/68 mmHg, SpO2 = 100%, and heart rate = 85 beats/min. Preoxygenation was performed with 100% oxygen. Volatile induction and maintenance anesthesia (VIMA) with sevoflurane, fentanyl (100 µg), and succinylcholine (75 mg) were used for induction. Direct laryngoscopy failed due to the severe tongue and neck swelling. After several attempts, she was orally intubated with a 6. 0 mm internal diameter reinforced endotracheal tube by video laryngoscope. Rocuronium 30 mg was then injected. Anesthesia had been maintained with 2 volume % of sevoflurane, 2 L/min of N2O, and 2 L/min of O2 (FiO2 0. 5). Volume controlled ventilation mode was used. The tidal volume was 400 ml, positive end expiratory pressure (PEEP) was 4 cmH2O, and the respiratory rate was 11 breaths/min.
In 40 minutes, peak inspiratory pressure increased from 22 cmH
2O to 38 cmH
2O and plateau pressure increased from 20 cmH
2O to 28 cmH
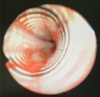
2O. We were not able to examine the endotracheal tube because her face was covered with surgical drapes. Since tidal volume was delivered, we managed to maintain ventilation within the normal range for the subsequent 40 minutes. At the end of the operation, we inspected the tube, but there was no kicking. On auscultation, weak breath sounds were heard from both lungs. We tried to pass a suction catheter through the endotracheal tube but it did not pass beyond 24 cm from the inlet. To determine the cause for obstruction, a fiberoptic bronchoscopic examination was conducted, and we recognized a longitudinal fold in the inner layer of the endotracheal tube forming a partial occlusion (
Fig. 1). Since prior direct laryngoscope had failed, we exchanged the reinforced tube with a 6. 0 mm internal diameter silastic tube using video laryngoscope to confirm visualization of the vocal cords (
Fig. 2). After replacement with a new Silastic tube, the peak inspiratory pressure and plateau pressure decreased to 22 cmH
2O and 20 cmH
2O, respectively. She was transferred to the intensive care unit with a T-piece.
DISCUSSION
Similar cases of dissection between the inner and outer layers of a reinforced endotracheal tube were reported in the literature (
Table 1). Most of them were related to repetitive resterilization with N
2O [
23] or even without N
2O [
45]. In some cases, the dissection was attributed to faulty manufacturing [
6789], while one case claimed that it was due to difficulty with stylet removal [
10]. During production of the tube, a liquid polyvinyl chloride layer coats a rod. The spiral steel wire was lodged in the rod. The coating process is repeated many times, creating the outer layer [
5]. Formation of air bubbles in the wall of a reinforced endotracheal tube can occur during production. The exposure to heat, such as during autoclaving, could create a separation in the inner layer, also leading to the formation of an air bubble. Additionally, the use of N
2O may expand it.
We assume that our case was related to the resterilization process. However, our center does not reuse tubes; the tube used in this case had not been used by other patients and was only resterilized with ethylene oxide.
In addition to resterilization, our case had other complex problems. A metal stylet was used because of a difficult airway. N
2O was used for general anesthesia. We reasoned that the following contributed to the dissection of the reinforced endotracheal tube: first, the resterilization may have created an air bubble between the layers; second, additional damage to the inner layer may have been inflicted by the metal stylet during its insertion or removal; and third, the air bubble increased during general anesthesia due to the use of N
2O (
Fig. 3).
There are several causes for an increase in peak inspiratory pressure, including the patient biting on the tube [
11], kinking of the tube [
12], internal obstruction by secretions, a pneumothorax, and bronchospasms. These should be considered in the differential diagnosis. Peak inspiratory pressure and plateau pressure are related to airway resistance and lung compliance, respectively. The difference between the peak and plateau pressures represents the pressure needed to overcome the resistance to airflow [
13]. We think that the resistant pressure of 10 cmH
2O (38 cmH
2O - 28 cmH
2O) was airway resistance caused by the obstruction of the dissected tube. If there is a sudden increase in peak inspiratory pressure, our recommendation is as follows: First, inspect for kinking of the tube. If there is no problem with the external tube, stop N
2O and give the patient 100% oxygen. Then, pass a suction catheter through the tube. If it cannot pass through the tube, perform a fiberoptic bronchoscope for direct internal visualization of the tube [
39].
In conclusion, ultimately, the use of a resterilized reinforced endotracheal tube must be avoided. Due to the possibility of faulty manufacturing, strict examination of the tube prior to use is highly recommended. When using a metal stylet, insertion and removal of the stylet should be performed with care. Since there is a recent trend towards the use of inhalation anesthetics, such as sevoflurane and desflurane, but not N2O, combined with short-acting opioids for general anesthesia, remifentanil is recommended instead of N2O.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download