References
2. Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004; 351:1764–1771.
4. Ministry of Health & Welfare. Korea Central Cancer Registry. National Cancer Center. Annual report of cancer statistics in Korea in 2013. Goyang: National Cancer Center;2015.
5. Kim WB, Kim TY, Kwon HS, Moon WJ, Lee JB, Choi YS, et al. Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer. J Korean Endocr Soc. 2007; 22:157–187.

6. Yi KH, Park YJ, Koong SS, Kim JH, Na DG, Ryu JS, et al. Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Endocrinol Metab. 2010; 25:270–297.

7. Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016; 9:59–126.

8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

9. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.

10. Dralle H, Musholt TJ, Schabram J, Steinmuller T, Frilling A, Simon D, et al. German association of endocrine surgeons practice guideline for the surgical management of malignant thyroid tumors. Langenbecks Arch Surg. 2013; 398:347–375.

11. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American association of clinical endocrinologists, associazione medici endocrinologi, and European thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest. 2010; 33:5 Suppl. 1–50.
12. Guerrier B, Berthet JP, Cartier C, Dehesdin D, Edet-Sanson A, Le Clech G, et al. French ENT Society (SFORL) practice guidelines for lymph-node management in adult differentiated thyroid carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2012; 129:197–206.

13. Pacini F, Castagna MG, Brilli L, Pentheroudakis G; ESMO Guidelines Working Group. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23:Suppl 7. vii110–vii119.

14. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81:Suppl 1. 1–122.

15. Pitoia F, Ward L, Wohllk N, Friguglietti C, Tomimori E, Gauna A, et al. Recommendations of the Latin American thyroid society on diagnosis and management of differentiated thyroid cancer. Arq Bras Endocrinol Metabol. 2009; 53:884–887.

16. Takami H, Ito Y, Okamoto T, Onoda N, Noguchi H, Yoshida A. Revisiting the guidelines issued by the Japanese society of thyroid surgeons and Japan association of endocrine surgeons: a gradual move towards consensus between Japanese and western practice in the management of thyroid carcinoma. World J Surg. 2014; 38:2002–2010.

17. Wemeau JL, Sadoul JL, d'Herbomez M, Monpeyssen H, Tramalloni J, Leteurtre E, et al. Guidelines of the French society of endocrinology for the management of thyroid nodules. Ann Endocrinol (Paris). 2011; 72:251–281.

18. Yi KH, Park YJ, Koong SS, Kim JH, Na DG, Ryu JS, et al. Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Korean J Otorhinolaryngol-Head Neck Surg. 2011; 54:8–36.

19. National Cancer Comprehensive Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Thyroid Carcinoma [Internet]. Washington: NCCN;2014. cited 2016
Nov 1. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
20. Pazaitou-Panayiotou K, Capezzone M, Pacini F. Clinical features and therapeutic implication of papillary thyroid microcarcinoma. Thyroid. 2007; 17:1085–1092.

21. Baudin E, Travagli JP, Ropers J, Mancusi F, Bruno-Bossio G, Caillou B, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998; 83:553–559.
22. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–428.

23. Verburg FA, Mader U, Luster M, Reiners C. Primary tumour diameter as a risk factor for advanced disease features of differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2009; 71:291–297.

24. Roti E, degli Uberti EC, Bondanelli M, Braverman LE. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008; 159:659–673.

25. Davies L, Welch HG. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg. 2010; 136:440–444.
26. Huang TW, Lai JH, Wu MY, Chen SL, Wu CH, Tam KW. Systematic review of clinical practice guidelines in the diagnosis and management of thyroid nodules and cancer. BMC Med. 2013; 11:191.

27. Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008; 32:747–753.

28. Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle RM. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid. 2014; 24:245–253.

29. Zheng X, Wei S, Han Y, Li Y, Yu Y, Yun X, et al. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol. 2013; 20:2266–2273.

30. Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, et al. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab. 2014; 99:2834–2843.

31. Lee J, Park JH, Lee CR, Chung WY, Park CS. Long-term outcomes of total thyroidectomy versus thyroid lobectomy for papillary thyroid microcarcinoma: comparative analysis after propensity score matching. Thyroid. 2013; 23:1408–1415.

32. Moon HJ, Kim EK, Chung WY, Yoon JH, Kwak JY. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: is it a real prognostic factor? Ann Surg Oncol. 2011; 18:1916–1923.

33. Lee YS, Lim H, Chang HS, Park CS. Papillary thyroid microcarcinomas are different from latent papillary thyroid carcinomas at autopsy. J Korean Med Sci. 2014; 29:676–679.

34. Kim DW, Lee EJ, Kim SH, Kim TH, Lee SH, Kim DH, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules: comparison in efficacy according to nodule size. Thyroid. 2009; 19:27–31.

35. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014; 24:27–34.

36. Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996; 5:43–63.

37. Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994; 18:559–567. discussion 567-8.

38. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003; 13:381–387.

39. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003; 98:31–40.

40. Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf). 2004; 60:21–28.

41. Solorzano CC, Carneiro DM, Ramirez M, Lee TM, Irvin GL 3rd. Surgeon-performed ultrasound in the management of thyroid malignancy. Am Surg. 2004; 70:576–580. discussion 580-2.
42. Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998; 29:4–10.

43. Jeong HS, Baek CH, Son YI, Choi JY, Kim HJ, Ko YH, et al. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf). 2006; 65:402–407.

44. Shetty SK, Maher MM, Hahn PF, Halpern EF, Aquino SL. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol. 2006; 187:1349–1356.

45. Razek AA, Sadek AG, Kombar OR, Elmahdy TE, Nada N. Role of apparent diffusion coefficient values in differentiation between malignant and benign solitary thyroid nodules. AJNR Am J Neuroradiol. 2008; 29:563–568.

46. Weber AL, Randolph G, Aksoy FG. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am. 2000; 38:1105–1129.
47. Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005; 90:5566–5575.

48. Dralle H, Sekulla C, Lorenz K, Brauckhoff M, Machens A;. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg. 2008; 32:1358–1366.

49. Chiang FY, Lu IC, Kuo WR, Lee KW, Chang NC, Wu CW. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery. 2008; 143:743–749.

50. Timmermann W, Hamelmann WH, Thomusch O, Sekulla C, Grond S, Neumann HJ, et al. Effectiveness and results of intraoperative neuromonitoring in thyroid surgery. Statement of the Interdisciplinary Study Group on Intraoperative Neuromonitoring of Thyroid Surgery. Chirurg. 2004; 75:916–922.

51. Thomusch O, Sekulla C, Machens A, Neumann HJ, Timmermann W, Dralle H. Validity of intra-operative neuromonitoring signals in thyroid surgery. Langenbecks Arch Surg. 2004; 389:499–503.

52. Hermann M, Hellebart C, Freissmuth M. Neuromonitoring in thyroid surgery: prospective evaluation of intraoperative electrophysiological responses for the prediction of recurrent laryngeal nerve injury. Ann Surg. 2004; 240:9–17.
53. Yarbrough DE, Thompson GB, Kasperbauer JL, Harper CM, Grant CS. Intraoperative electromyographic monitoring of the recurrent laryngeal nerve in reoperative thyroid and parathyroid surgery. Surgery. 2004; 136:1107–1115.

54. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998; 228:320–330.

55. Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope. 2013; 123:2056–2063.

56. Gourin CG, Tufano RP, Forastiere AA, Koch WM, Pawlik TM, Bristow RE. Volume-based trends in thyroid surgery. Arch Otolaryngol Head Neck Surg. 2010; 136:1191–1198.

57. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007; 246:375–381.

58. Grant CS, Hay ID, Gough IR, Bergstralh EJ, Goellner JR, McConahey WM. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. 1988; 104:954–962.
59. Hay ID, Grant CS, Bergstralh EJ, Thompson GB, van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998; 124:958–964.

60. Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001; 86:1447–1463.

61. Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014; 38:68–79.

62. Barney BM, Hitchcock YJ, Sharma P, Shrieve DC, Tward JD. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck. 2011; 33:645–649.

63. Mendelsohn AH, Elashoff DA, Abemayor E, St John MA. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010; 136:1055–1061.
64. Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005; 12:81–89.

65. Nixon IJ, Ganly I, Patel SG, Palmer FL, Whitcher MM, Tuttle RM, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012; 151:571–579.

66. Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992; 75:714–720.

67. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993; 114:1050–1057. discussion 1057-8.
68. Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002; 26:879–885.

69. Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998; 133:419–425.
70. Gimm O, Rath FW, Dralle H. Pattern of lymph node metastases in papillary thyroid carcinoma. Br J Surg. 1998; 85:252–254.

71. Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002; 131:249–256.

72. DeGroot LJ. Long-term impact of initial and surgical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:499–500.

73. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005; 71:731–734.

74. Conzo G, Docimo G, Mauriello C, Gambardella C, Esposito D, Cavallo F, et al. The current status of lymph node dissection in the treatment of papillary thyroid cancer. A literature review. Clin Ter. 2013; 164:e343–e346.
75. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008; 144:1070–1077. discussion 1077-8.

76. Yang L, Shen W, Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol. 2013; 31:468–474.

77. Tisell LE, Nilsson B, Molne J, Hansson G, Fjalling M, Jansson S, et al. Improved survival of patients with papillary thyroid cancer after surgical microdissection. World J Surg. 1996; 20:854–859.

78. Barczyński M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. 2013; 100:410–418.

79. Costa S, Giugliano G, Santoro L, Ywata De, Massaro MA, Gibelli B, et al. Role of prophylactic central neck dissection in cN0 papillary thyroid cancer. Acta Otorhinolaryngol Ital. 2009; 29:61–69.
80. Moo TA, McGill J, Allendorf J, Lee J, Fahey T 3rd, Zarnegar R. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg. 2010; 34:1187–1191.

81. Moreno MA, Edeiken-Monroe BS, Siegel ER, Sherman SI, Clayman GL. In papillary thyroid cancer, preoperative central neck ultrasound detects only macroscopic surgical disease, but negative findings predict excellent long-term regional control and survival. Thyroid. 2012; 22:347–355.

82. Wang TS, Evans DB, Fareau GG, Carroll T, Yen TW. Effect of prophylactic central compartment neck dissection on serum thyroglobulin and recommendations for adjuvant radioactive iodine in patients with differentiated thyroid cancer. Ann Surg Oncol. 2012; 19:4217–4222.

83. Hartl DM, Leboulleux S, Al Ghuzlan A, Baudin E, Chami L, Schlumberger M, et al. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg. 2012; 255:777–783.

84. Baek SK, Jung KY, Kang SM, Kwon SY, Woo JS, Cho SH, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010; 20:147–152.

85. Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011; 150:1048–1057.

86. Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012; 36:1274–1278.

87. Sancho JJ, Lennard TW, Paunovic I, Triponez F, Sitges-Serra A. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg. 2014; 399:155–163.

88. Cheah WK, Arici C, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Complications of neck dissection for thyroid cancer. World J Surg. 2002; 26:1013–1016.

89. Lang BH, Ng SH, Lau LL, Cowling BJ, Wong KP, Wan KY. A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid. 2013; 23:1087–1098.

90. Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol. 2013; 20:3477–3483.

91. Raffaelli M, De Crea C, Sessa L, Giustacchini P, Revelli L, Bellantone C, et al. Prospective evaluation of total thyroidectomy versus ipsilateral versus bilateral central neck dissection in patients with clinically node-negative papillary thyroid carcinoma. Surgery. 2012; 152:957–964.

92. Lee KE, Chung IY, Kang E, Koo do H, Kim KH, Kim SW, et al. Ipsilateral and contralateral central lymph node metastasis in papillary thyroid cancer: patterns and predictive factors of nodal metastasis. Head Neck. 2013; 35:672–676.

93. Koo BS, Choi EC, Yoon YH, Kim DH, Kim EH, Lim YC. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg. 2009; 249:840–844.

94. Sadowski BM, Snyder SK, Lairmore TC. Routine bilateral central lymph node clearance for papillary thyroid cancer. Surgery. 2009; 146:696–703. discussion 703-5.

95. Gemsenjager E, Perren A, Seifert B, Schuler G, Schweizer I, Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003; 197:182–190.

96. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004; 28:498–501.

97. Farrag T, Lin F, Brownlee N, Kim M, Sheth S, Tufano RP. Is routine dissection of level II-B and V-A necessary in patients with papillary thyroid cancer undergoing lateral neck dissection for FNA-confirmed metastases in other levels. World J Surg. 2009; 33:1680–1683.

98. Koo BS, Yoon YH, Kim JM, Choi EC, Lim YC. Predictive factors of level IIb lymph node metastasis in patients with papillary thyroid carcinoma. Ann Surg Oncol. 2009; 16:1344–1347.

99. Kupferman ME, Patterson DM, Mandel SJ, LiVolsi V, Weber RS. Safety of modified radical neck dissection for differentiated thyroid carcinoma. Laryngoscope. 2004; 114:403–406.

100. Goropoulos A, Karamoshos K, Christodoulou A, Ntitsias T, Paulou K, Samaras A, et al. Value of the cervical compartments in the surgical treatment of papillary thyroid carcinoma. World J Surg. 2004; 28:1275–1281.

101. Lee CR, Nam KH. Lateral neck node dissection in differentiated thyroid carcinoma. Korean J Endocr Surg. 2014; 14:1–6.

102. Ducoudray R, Tresallet C, Godiris-Petit G, Tissier F, Leenhardt L, Menegaux F. Prophylactic lymph node dissection in papillary thyroid carcinoma: is there a place for lateral neck dissection? World J Surg. 2013; 37:1584–1591.

103. Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007; 13:498–512.

104. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012; 22:1144–1152.

105. Lee J, Song Y, Soh EY. Prognostic significance of the number of metastatic lymph nodes to stratify the risk of recurrence. World J Surg. 2014; 38:858–862.

106. Vaisman F, Shaha A, Fish S, Michael Tuttle R. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). 2011; 75:112–119.

107. Erdem E, Gulcelik MA, Kuru B, Alagol H. Comparison of completion thyroidectomy and primary surgery for differentiated thyroid carcinoma. Eur J Surg Oncol. 2003; 29:747–749.

108. Tan MP, Agarwal G, Reeve TS, Barraclough BH, Delbridge LW. Impact of timing on completion thyroidectomy for thyroid cancer. Br J Surg. 2002; 89:802–804.

109. Untch BR, Palmer FL, Ganly I, Patel SG, Michael Tuttle R, Shah JP, et al. Oncologic outcomes after completion thyroidectomy for patients with well-differentiated thyroid carcinoma. Ann Surg Oncol. 2014; 21:1374–1378.

110. Barbesino G, Goldfarb M, Parangi S, Yang J, Ross DS, Daniels GH. Thyroid lobe ablation with radioactive iodine as an alternative to completion thyroidectomy after hemithyroidectomy in patients with follicular thyroid carcinoma: long-term follow-up. Thyroid. 2012; 22:369–376.

111. Santra A, Bal S, Mahargan S, Bal C. Long-term outcome of lobar ablation versus completion thyroidectomy in differentiated thyroid cancer. Nucl Med Commun. 2011; 32:52–58.

112. Giovanella L, Piccardo A, Paone G, Foppiani L, Treglia G, Ceriani L. Thyroid lobe ablation with iodine-131I in patients with differentiated thyroid carcinoma: a randomized comparison between 1.1 and 3.7 GBq activities. Nucl Med Commun. 2013; 34:767–770.

113. Kebebew E, Clark OH. Locally advanced differentiated thyroid cancer. Surg Oncol. 2003; 12:91–99.

114. Musholt TJ, Musholt PB, Behrend M, Raab R, Scheumann GF, Klempnauer J. Invasive differentiated thyroid carcinoma: tracheal resection and reconstruction procedures in the hands of the endocrine surgeon. Surgery. 1999; 126:1078–1087. discussion 1087-8.

115. Brauckhoff M, Meinicke A, Bilkenroth U, Lorenz K, Brauckhoff K, Gimm O, et al. Long-term results and functional outcome after cervical evisceration in patients with thyroid cancer. Surgery. 2006; 140:953–959.

116. Brauckhoff M, Machens A, Thanh PN, Lorenz K, Schmeil A, Stratmann M, et al. Impact of extent of resection for thyroid cancer invading the aerodigestive tract on surgical morbidity, local recurrence, and cancer-specific survival. Surgery. 2010; 148:1257–1266.

117. Czaja JM, McCaffrey TV. The surgical management of laryngotracheal invasion by well-differentiated papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 1997; 123:484–490.

118. Ark N, Zemo S, Nolen D, Holsinger FC, Weber RS. Management of locally invasive well-differentiated thyroid cancer. Surg Oncol Clin N Am. 2008; 17:145–155. ix

119. Nishida T, Nakao K, Hamaji M, Kamiike W, Kurozumi K, Matsuda H. Preservation of recurrent laryngeal nerve invaded by differentiated thyroid cancer. Ann Surg. 1997; 226:85–91.

120. McCaffrey TV, Bergstralh EJ, Hay ID. Locally invasive papillary thyroid carcinoma: 1940-1990. Head Neck. 1994; 16:165–172.

121. Gillenwater AM, Goepfert H. Surgical management of laryngotracheal and esophageal involvement by locally advanced thyroid cancer. Semin Surg Oncol. 1999; 16:19–29.

122. Cody HS 3rd, Shah JP. Locally invasive, well-differentiated thyroid cancer. 22 years' experience at Memorial Sloan-Kettering Cancer Center. Am J Surg. 1981; 142:480–483.
123. Tsai YF, Tseng YL, Wu MH, Hung CJ, Lai WW, Lin MY. Aggressive resection of the airway invaded by thyroid carcinoma. Br J Surg. 2005; 92:1382–1387.

124. Djalilian M, Beahrs OH, Devine KD, Weiland LH, DeSanto LW. Intraluminal involvement of the larynx and trachea by thyroid cancer. Am J Surg. 1974; 128:500–504.

125. Honings J, Stephen AE, Marres HA, Gaissert HA. The management of thyroid carcinoma invading the larynx or trachea. Laryngoscope. 2010; 120:682–689.

126. Friedman M. Surgical management of thyroid carcinoma with laryngotracheal invasion. Otolaryngol Clin North Am. 1990; 23:495–507.

127. Grillo HC, Zannini P. Resectional management of airway invasion by thyroid carcinoma. Ann Thorac Surg. 1986; 42:287–298.

128. Grillo HC, Suen HC, Mathisen DJ, Wain JC. Resectional management of thyroid carcinoma invading the airway. Ann Thorac Surg. 1992; 54:3–9.

129. Madsen JC, Mathisen DJ, Grillo HC. Cervical exenteration. Semin Thorac Cardiovasc Surg. 1992; 4:292–299.
130. Patel KN, Shaha AR. Locally advanced thyroid cancer. Curr Opin Otolaryngol Head Neck Surg. 2005; 13:112–116.

131. Falk SA, McCaffrey TV. Management of the recurrent laryngeal nerve in suspected and proven thyroid cancer. Otolaryngol Head Neck Surg. 1995; 113:42–48.

132. Chiang FY, Lin JC, Lee KW, Wang LF, Tsai KB, Wu CW, et al. Thyroid tumors with preoperative recurrent laryngeal nerve palsy: clinicopathologic features and treatment outcome. Surgery. 2006; 140:413–417.

133. Wilson PC, Millar BM, Brierley JD. The management of advanced thyroid cancer. Clin Oncol (R Coll Radiol). 2004; 16:561–568.

134. Nishida T, Nakao K, Hamaji M. Differentiated thyroid carcinoma with airway invasion: indication for tracheal resection based on the extent of cancer invasion. J Thorac Cardiovasc Surg. 1997; 114:84–92.

135. Machens A, Hinze R, Lautenschlager C, Thomusch O, Dralle H. Thyroid carcinoma invading the cervicovisceral axis: routes of invasion and clinical implications. Surgery. 2001; 129:23–28.

136. Mazzaferri EL, Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002; 9:227–247.

137. Kasperbauer JL. Locally advanced thyroid carcinoma. Ann Otol Rhinol Laryngol. 2004; 113:749–753.

138. Brierley JD, Tsang RW. External radiation therapy in the treatment of thyroid malignancy. Endocrinol Metab Clin North Am. 1996; 25:141–157.

139. Tubiana M, Haddad E, Schlumberger M, Hill C, Rougier P, Sarrazin D. External radiotherapy in thyroid cancers. Cancer. 1985; 55:9 Suppl. 2062–2071.

140. Farahati J, Reiners C, Stuschke M, Muller SP, Stuben G, Sauerwein W, et al. Differentiated thyroid cancer. Impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration (stage pT4). Cancer. 1996; 77:172–180.

141. Kouvaraki MA, Shapiro SE, Fornage BD, Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003; 134:946–954. discussion 954-5.

142. Torlontano M, Crocetti U, Augello G, D'Aloiso L, Bonfitto N, Varraso A, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006; 91:60–63.

143. Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88:3668–3673.

144. Snozek CL, Chambers EP, Reading CC, Sebo TJ, Sistrunk JW, Singh RJ, et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab. 2007; 92:4278–4281.

145. Cunha N, Rodrigues F, Curado F, Ilheu O, Cruz C, Naidenov P, et al. Thyroglobulin detection in fine-needle aspirates of cervical lymph nodes: a technique for the diagnosis of metastatic differentiated thyroid cancer. Eur J Endocrinol. 2007; 157:101–107.

146. Bachelot A, Cailleux AF, Klain M, Baudin E, Ricard M, Bellon N, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002; 12:707–711.

147. David A, Blotta A, Bondanelli M, Rossi R, Roti E, Braverman LE, et al. Serum thyroglobulin concentrations and (131)I whole-body scan results in patients with differentiated thyroid carcinoma after administration of recombinant human thyroid-stimulating hormone. J Nucl Med. 2001; 42:1470–1475.
148. Wang W, Larson SM, Fazzari M, Tickoo SK, Kolbert K, Sgouros G, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000; 85:1107–1113.

149. Saghari M, Gholamrezanezhad A, Mirpour S, Eftekhari M, Takavar A, Fard-Esfahani A, et al. Efficacy of radioiodine therapy in the treatment of elevated serum thyroglobulin in patients with differentiated thyroid carcinoma and negative whole-body iodine scan. Nucl Med Commun. 2006; 27:567–572.

150. Alzahrani AS, Mohamed G, Al Shammary A, Aldasouqi S, Abdal Salam S, Shoukri M. Long-term course and predictive factors of elevated serum thyroglobulin and negative diagnostic radioiodine whole body scan in differentiated thyroid cancer. J Endocrinol Invest. 2005; 28:540–546.

151. Chao M. Management of differentiated thyroid cancer with rising thyroglobulin and negative diagnostic radioiodine whole body scan. Clin Oncol (R Coll Radiol). 2010; 22:438–447.

152. Al-Saif O, Farrar WB, Bloomston M, Porter K, Ringel MD, Kloos RT. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab. 2010; 95:2187–2194.

153. Yim JH, Kim WB, Kim EY, Kim WG, Kim TY, Ryu JS, et al. The outcomes of first reoperation for locoregionally recurrent/ persistent papillary thyroid carcinoma in patients who initially underwent total thyroidectomy and remnant ablation. J Clin Endocrinol Metab. 2011; 96:2049–2056.

154. Schuff KG, Weber SM, Givi B, Samuels MH, Andersen PE, Cohen JI. Efficacy of nodal dissection for treatment of persistent/ recurrent papillary thyroid cancer. Laryngoscope. 2008; 118:768–775.

155. Clayman GL, Shellenberger TD, Ginsberg LE, Edeiken BS, El-Naggar AK, Sellin RV, et al. Approach and safety of comprehensive central compartment dissection in patients with recurrent papillary thyroid carcinoma. Head Neck. 2009; 31:1152–1163.

156. Tufano RP, Clayman G, Heller KS, Inabnet WB, Kebebew E, Shaha A, et al. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid. 2015; 25:15–27.

157. Rosenthal MS, Angelos P, Cooper DS, Fassler C, Finder SG, Hays MT, et al. Clinical and professional ethics guidelines for the practice of thyroidology. Thyroid. 2013; 23:1203–1210.

158. Rondeau G, Fish S, Hann LE, Fagin JA, Tuttle RM. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid. 2011; 21:845–853.

159. Robenshtok E, Fish S, Bach A, Dominguez JM, Shaha A, Tuttle RM. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab. 2012; 97:2706–2713.

160. Lim HK, Baek JH, Lee JH, Kim WB, Kim TY, Shong YK, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015; 25:163–170.

161. Wang L, Ge M, Xu D, Chen L, Qian C, Shi K, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Cancer Res Ther. 2014; 10:C144–C149.
162. Wu G, Fraser S, Pai SI, Farrag TY, Ladenson PW, Tufano RP. Determining the extent of lateral neck dissection necessary to establish regional disease control and avoid reoperation after previous total thyroidectomy and radioactive iodine for papillary thyroid cancer. Head Neck. 2012; 34:1418–1421.

163. Stack BC Jr, Ferris RL, Goldenberg D, Haymart M, Shaha A, Sheth S, et al. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid. 2012; 22:501–508.

164. Lee L, Steward DL. Sonographically-directed neck dissection for recurrent thyroid carcinoma. Laryngoscope. 2008; 118:991–994.

165. Farrag TY, Agrawal N, Sheth S, Bettegowda C, Ewertz M, Kim M, et al. Algorithm for safe and effective reoperative thyroid bed surgery for recurrent/persistent papillary thyroid carcinoma. Head Neck. 2007; 29:1069–1074.

166. Roh JL, Park JY, Rha KS, Park CI. Is central neck dissection necessary for the treatment of lateral cervical nodal recurrence of papillary thyroid carcinoma? Head Neck. 2007; 29:901–906.

167. Ronga G, Filesi M, Montesano T, Di Nicola AD, Pace C, Travascio L, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years' experience. Q J Nucl Med Mol Imaging. 2004; 48:12–19.
168. Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996; 37:598–605.
169. Ilgan S, Karacalioglu AO, Pabuscu Y, Atac GK, Arslan N, Ozturk E, et al. Iodine-131 treatment and high-resolution CT: results in patients with lung metastases from differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004; 31:825–830.

170. Hod N, Hagag P, Baumer M, Sandbank J, Horne T. Differentiated thyroid carcinoma in children and young adults: evaluation of response to treatment. Clin Nucl Med. 2005; 30:387–390.

171. Koh JM, Kim ES, Ryu JS, Hong SJ, Kim WB, Shong YK. Effects of therapeutic doses of 131I in thyroid papillary carcinoma patients with elevated thyroglobulin level and negative 131I whole-body scan: comparative study. Clin Endocrinol (Oxf). 2003; 58:421–427.

172. Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001; 86:1568–1573.

173. Zettinig G, Fueger BJ, Passler C, Kaserer K, Pirich C, Dudczak R, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma -- surgery or conventional therapy? Clin Endocrinol (Oxf). 2002; 56:377–382.

174. Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997; 82:3637–3642.

175. McWilliams RR, Giannini C, Hay ID, Atkinson JL, Stafford SL, Buckner JC. Management of brain metastases from thyroid carcinoma: a study of 16 pathologically confirmed cases over 25 years. Cancer. 2003; 98:356–362.

176. Hong HJ, Kim WS, Koh YW, Lee SY, Shin YS, Koo YC, et al. Endoscopic thyroidectomy via an axillo-breast approach without gas insufflation for benign thyroid nodules and micropapillary carcinomas: preliminary results. Yonsei Med J. 2011; 52:643–654.

177. Lee H, Lee J, Sung KY. Comparative study comparing endoscopic thyroidectomy using the axillary approach and open thyroidectomy for papillary thyroid microcarcinoma. World J Surg Oncol. 2012; 10:269.

178. Tae K, Ji YB, Cho SH, Kim KR, Kim DW, Kim DS. Initial experience with a gasless unilateral axillo-breast or axillary approach endoscopic thyroidectomy for papillary thyroid microcarcinoma: comparison with conventional open thyroidectomy. Surg Laparosc Endosc Percutan Tech. 2011; 21:162–169.

179. Chung YS, Choe JH, Kang KH, Kim SW, Chung KW, Park KS, et al. Endoscopic thyroidectomy for thyroid malignancies: comparison with conventional open thyroidectomy. World J Surg. 2007; 31:2302–2306.

180. Hyun K, Byon W, Park HJ, Park Y, Park C, Yun JS. Comparison of swallowing disorder following gasless transaxillary endoscopic thyroidectomy versus conventional open thyroidectomy. Surg Endosc. 2014; 28:1914–1920.

181. Jeong JJ, Kang SW, Yun JS, Sung TY, Lee SC, Lee YS, et al. Comparative study of endoscopic thyroidectomy versus conventional open thyroidectomy in papillary thyroid microcarcinoma (PTMC) patients. J Surg Oncol. 2009; 100:477–480.

182. Im HJ, Koo do H, Paeng JC, Lee KE, Chung YS, Lim I, et al. Evaluation of surgical completeness in endoscopic thyroidectomy compared with open thyroidectomy with regard to remnant ablation. Clin Nucl Med. 2012; 37:148–151.

183. Son SK, Kim JH, Bae JS, Lee SH. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2015; 22:3022–3032.

184. Shen H, Shan C, Qiu M. Systematic review and meta-analysis of transaxillary robotic thyroidectomy versus open thyroidectomy. Surg Laparosc Endosc Percutan Tech. 2014; 24:199–206.

185. Sun GH, Peress L, Pynnonen MA. Systematic review and meta-analysis of robotic vs conventional thyroidectomy approaches for thyroid disease. Otolaryngol Head Neck Surg. 2014; 150:520–532.

186. Lang BH, Wong CK, Tsang JS, Wong KP, Wan KY. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol. 2014; 21:850–861.

187. Jackson NR, Yao L, Tufano RP, Kandil EH. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head Neck. 2014; 36:137–143.

188. Noureldine SI, Abdelghani R, Saeed A, Cortes N, Abbas A, Aslam R, et al. Is robotic hemithyroidectomy comparable to its conventional counterpart? Surgery. 2013; 154:363–368.

189. Kwak HY, Kim HY, Lee HY, Jung SP, Woo SU, Son GS, et al. Robotic thyroidectomy using bilateral axillo-breast approach: Comparison of surgical results with open conventional thyroidectomy. J Surg Oncol. 2015; 111:141–145.

190. Kim BS, Kang KH, Kang H, Park SJ. Central neck dissection using a bilateral axillo-breast approach for robotic thyroidectomy: comparison with conventional open procedure after propensity score matching. Surg Laparosc Endosc Percutan Tech. 2014; 24:67–72.

191. Yi O, Yoon JH, Lee YM, Sung TY, Chung KW, Kim TY, et al. Technical and oncologic safety of robotic thyroid surgery. Ann Surg Oncol. 2013; 20:1927–1933.

192. Tae K, Song CM, Ji YB, Kim KR, Kim JY, Choi YY. Comparison of surgical completeness between robotic total thyroidectomy versus open thyroidectomy. Laryngoscope. 2014; 124:1042–1047.

193. Kim WW, Kim JS, Hur SM, Kim SH, Lee SK, Choi JH, et al. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World J Surg. 2011; 35:779–784.

194. Tae K, Ji YB, Cho SH, Lee SH, Kim DS, Kim TW. Early surgical outcomes of robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach for papillary thyroid carcinoma: 2 years' experience. Head Neck. 2012; 34:617–625.

195. Lee S, Ryu HR, Park JH, Kim KH, Kang SW, Jeong JJ, et al. Early surgical outcomes comparison between robotic and conventional open thyroid surgery for papillary thyroid microcarcinoma. Surgery. 2012; 151:724–730.

196. Tae K, Kim KY, Yun BR, Ji YB, Park CW, Kim DS, et al. Functional voice and swallowing outcomes after robotic thyroidectomy by a gasless unilateral axillo-breast approach: comparison with open thyroidectomy. Surg Endosc. 2012; 26:1871–1877.

197. Ryu HR, Lee J, Park JH, Kang SW, Jeong JJ, Hong JY, et al. A comparison of postoperative pain after conventional open thyroidectomy and transaxillary single-incision robotic thyroidectomy: a prospective study. Ann Surg Oncol. 2013; 20:2279–2284.

198. Lee J, Nah KY, Kim RM, Ahn YH, Soh EY, Chung WY. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc. 2010; 24:3186–3194.

199. Song CM, Ji YB, Bang HS, Park CW, Kim DS, Tae K. Quality of life after robotic thyroidectomy by a gasless unilateral axillary approach. Ann Surg Oncol. 2014; 21:4188–4194.

200. Lee S, Kim HY, Lee CR, Park S, Son H, Kang SW, et al. A prospective comparison of patient body image after robotic thyroidectomy and conventional open thyroidectomy in patients with papillary thyroid carcinoma. Surgery. 2014; 156:117–125.

201. Lee J, Na KY, Kim RM, Oh Y, Lee JH, Lee J, et al. Postoperative functional voice changes after conventional open or robotic thyroidectomy: a prospective trial. Ann Surg Oncol. 2012; 19:2963–2970.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



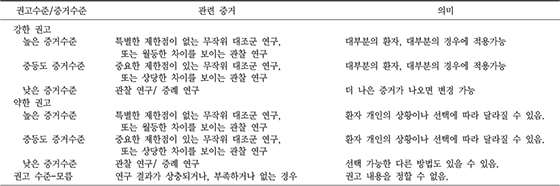

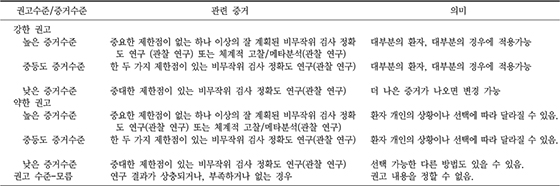
 XML Download
XML Download