Abstract
Purpose
Although papillary thyroid microcarcinoma (PTMC) has very excellent prognosis, lymph node metastases are found frequently. This study identifies the risk factors of clinically negative cervical lymph node metastasis (cN0) and investigates the need for central lymph node dissection in cN0 PTMC.
Methods
From Jan. 1st 2007 to Dec. 30th 2013, 1593 patients received surgery for papillary thyroid carcinoma. Seven hundred and eleven patients were diagnosed with cN0 PTMCs. They all received thyroidectomy (total thyroidectomy or lobectomy) with prophylactic central neck dissection. We reviewed the medical records and analyzed the risk factors affecting central lymph node metastasis (CLNM).
Results
Of 711 PTMCs patients without clinical lymph node metastasis, 170 (23.9%) patients had CLNM. CLNM was frequent in males than females (P<0.001). The larger the primary tumor, the higher the risk of CLNM (P<0.001). Extrathyroidal extension was an independent risk factor of CLNM (P<0.001). Recurrence rates in the CLNM negative group was 1.3%, and in the CLNM positive group 2.4%. The CLNM positive group recurred at a slightly higher rate, but not statistically significantly (P=0.329). Five year disease free survival in the CLNM negative was 96.8%, and in the positive group 94.1%, not a statistically significant (P=0.630).
Conclusion
In this study, male gender, the size of primary tumor, and Extrathyroidal extension were the risk factors of occult LNM but occult LNM in PTMC was not associated with recurrence rate or five-year disease free survivals. Therefore, we can omit prophylactic central lymph node dissection in patient with cN0 PTMC.
References
1. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989; 63:908–11.

2. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006; 295:2164–7.

3. Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrinol Metab. 2007; 3:240–8.

4. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–28.

5. Harach HR, Franssila KO. Occult papillary carcinoma of the thyroid appearing as lung metastasis. Arch Pathol Lab Med. 1984; 108:529–30.
6. Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006; 141:489–96.

7. Williams ED. Guest editorial: two proposals regarding the terminology of thyroid tumors. Int J Surg Pathol. 2000; 8:181–3.

8. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J. 1999; 46:209–16.

9. Lang W, Borrusch H, Bauer L. Occult carcinomas of the thyroid. Evaluation of 1,020 sequential autopsies. Am J Clin Pathol. 1988; 90:72–6.

10. Yamamoto Y, Maeda T, Izumi K, Otsuka H. Occult papillary carcinoma of the thyroid. A study of 408 autopsy cases. Cancer. 1990; 65:1173–9.

11. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

12. Attie JN, Setzin M, Klein I. Thyroid carcinoma presenting as an enlarged cervical lymph node. Am J Surg. 1993; 166:428–30.

13. Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008; 32:747–53.

Fig. 1.
Ultrasound images of central lymph node metastasis. (A) LNM with a loss of normal hilar echogenicity; (B) LNM with long diameter (>5 mm) and increased echogenicity; (C) LNM with round shape; (D) LNM with extracapsular metastasis. LNM = lymph node metastasis.

Fig. 2.
Recurrence-free survival curve. 5-year disease free survival in CLNM negative and positive group were 96.8% and 94.1%. It was not different statistically (P=0.630). CLNM = central lymph node metastasis.
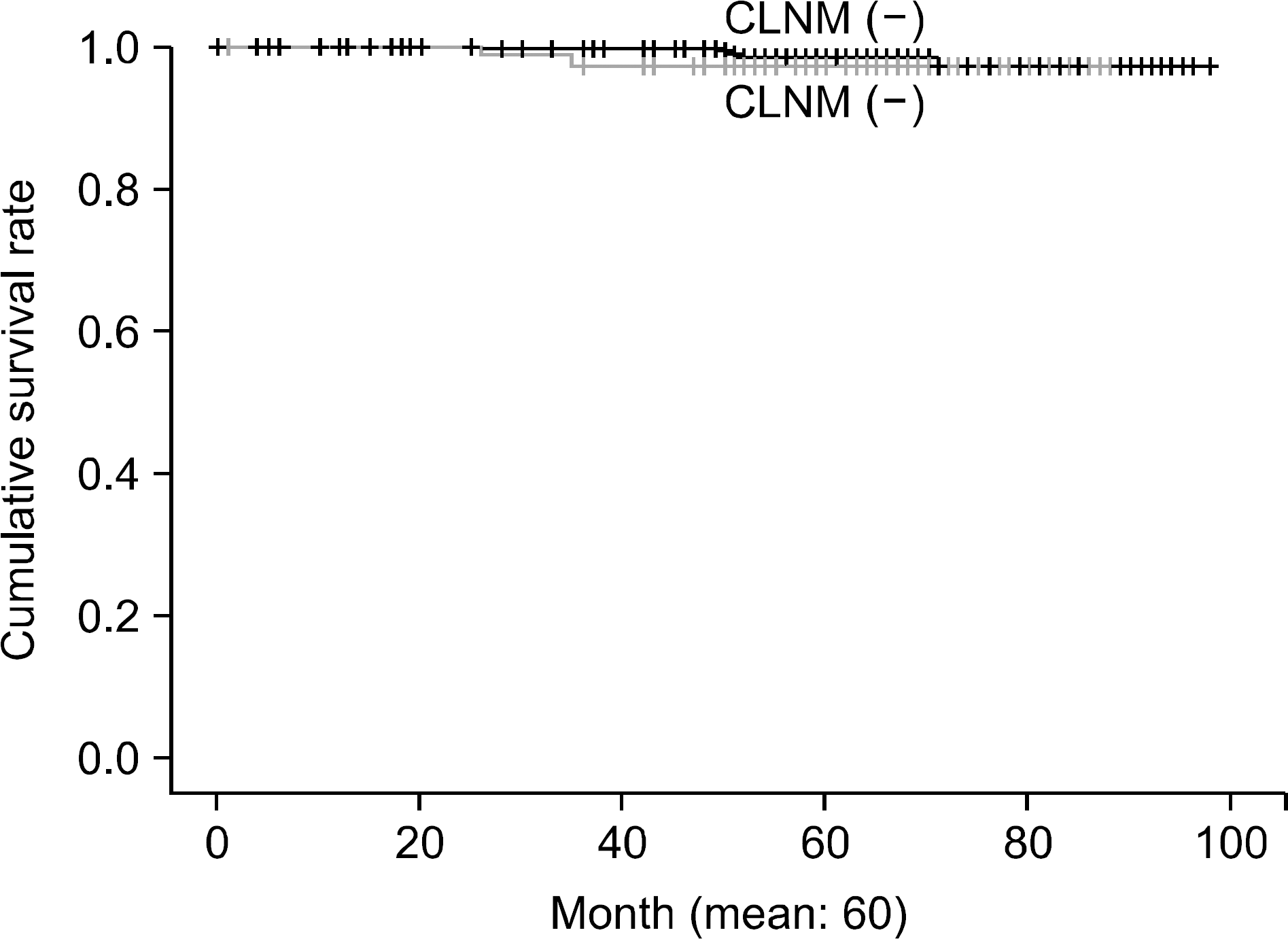
Table 1.
Characteristics of patients and tumors
Table 2.
Multivariate analysis of risk factors for clinically negative central lymph node metastasis
| OR | P value | 95% CI | |
|---|---|---|---|
| Age | 0.997 | 0.005 | 0.962∼0.993 |
| Sex Tumor size | 0.294 1.160 | 0.000 0.005 | 0.193∼0.450 1.045∼1.287 |
| ETE | 2.068 | 0.000 | 1.405∼3.043 |
Table 3.
Correlation of clinical and histological features with central neck metastasis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download