Abstract
Purpose
We assessed the prognostic value of AMES to determine the extent of surgery in PTC patients, and compared AMES score usefulness and accuracy with [18F] FDG PET/CT.
Methods
We conducted a review of data from a single center and a single surgeon, who treated 341 patients with PTC with total thyroidectomy and prophylactic bilateral CLN dissection at a tertiary referral center, Chungnam National University Hospital, between 2001 and 2012.
Results
In multivariate analysis, the rate of CLN metastasis was considerably higher in PTC patients with the higher AMES score (odds ratio [OR], 1.718; 95% confidence interval [CI], 1.073∼2.752), higher SUV of the CLN (>0) (OR, 6.525; CI, 3.184∼13.371), higher SUV of the tumor (>4.3) (OR, 1.855; CI, 1.065∼3.231).
References
1. Moo TA, McGill J, Allendorf J, Lee J, Fahey T 3rd, Zarnegar R. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg. 2010; 34:1187–91.

2. Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope. 2004; 114:393–402.

3. Lee YS, Kim SW, Kim SW, Kim SK, Kang HS, Lee ES, et al. Extent of routine central lymph node dissection with small papillary thyroid carcinoma. World J Surg. 2007; 31:1954–9.

4. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J. 1999; 46:209–16.

5. Hay ID, Grant CS, Bergstralh EJ, Thompson GB, van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998; 124:958–64.

6. Dionigi G, Dionigi R, Bartalena L, Boni L, Rovera F, Villa F. Surgery of lymph nodes in papillary thyroid cancer. Expert Rev Anticancer Ther. 2006; 6:1217–29.

7. Pellegriti G, Scollo C, Lumera G, Regalbuto C, Vigneri R, Belfiore A. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab. 2004; 89:3713–20.

8. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007; 246:375–81.

9. Kim H, Na KJ, Choi JH, Ahn BC, Ahn D, Sohn JH. Feasibility of FDG-PET/CT for the initial diagnosis of papillary thyroid cancer. Eur Arch Otorhinolaryngol 2015. [Epub ahead of print].
10. Wang W, Larson SM, Fazzari M, Tickoo SK, Kolbert K, Sgouros G, et al. Prognostic value of [18F] fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000; 85:1107–13.
11. Rossi RL, Cady B, Silverman ML, Wool MS, Horner TA. Current results of conservative surgery for differentiated thyroid carcinoma. World J Surg. 1986; 10:612–22.

12. Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988; 104:947–53.
13. Cady B. Hayes Martin Lecture. Our AMES is true: how an old concept still hits the mark: or, risk group assignment points the arrow to rational therapy selection in differentiated thyroid cancer. Am J Surg. 1997; 174:462–8.
14. Shah JP, Loree TR, Dharker D, Strong EW, Begg C, Vlamis V. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992; 164:658–61.

15. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993; 114:1050–7.
16. Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005; 12:81–9.

17. Roh JL, Kim JM, Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol. 2011; 18:2245–50.

18. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237:399–407.
19. Moo TA, Umunna B, Kato M, Butriago D, Kundel A, Lee JA, et al. Ipsilateral versus bilateral central neck lymph node dissection in papillary thyroid carcinoma. Ann Surg. 2009; 250:403–8.

20. Palestini N, Borasi A, Cestino L, Freddi M, Odasso C, Robecchi A. Is central neck dissection a safe procedure in the treatment of papillary thyroid cancer? Our experience. Langenbecks Arch Surg. 2008; 393:693–8.

21. Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996; 5:43–63.

22. Schindler AM, van Melle G, Evequoz B, Scazziga B. Prognostic factors in papillary carcinoma of the thyroid. Cancer. 1991; 68:324–30.

23. Tubiana M, Schlumberger M, Rougier P, Laplanche A, Benhamou E, Gardet P, et al. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer. 1985; 55:794–804.

Fig. 1.
Receiver operating characteristic curve of tumor SUVmax. With an SUVmax cutoff value of above 4.3, the surgeon can predict central lymph node (CLN) metastasis, and proceed total thyroidectomy and CLN dissection.
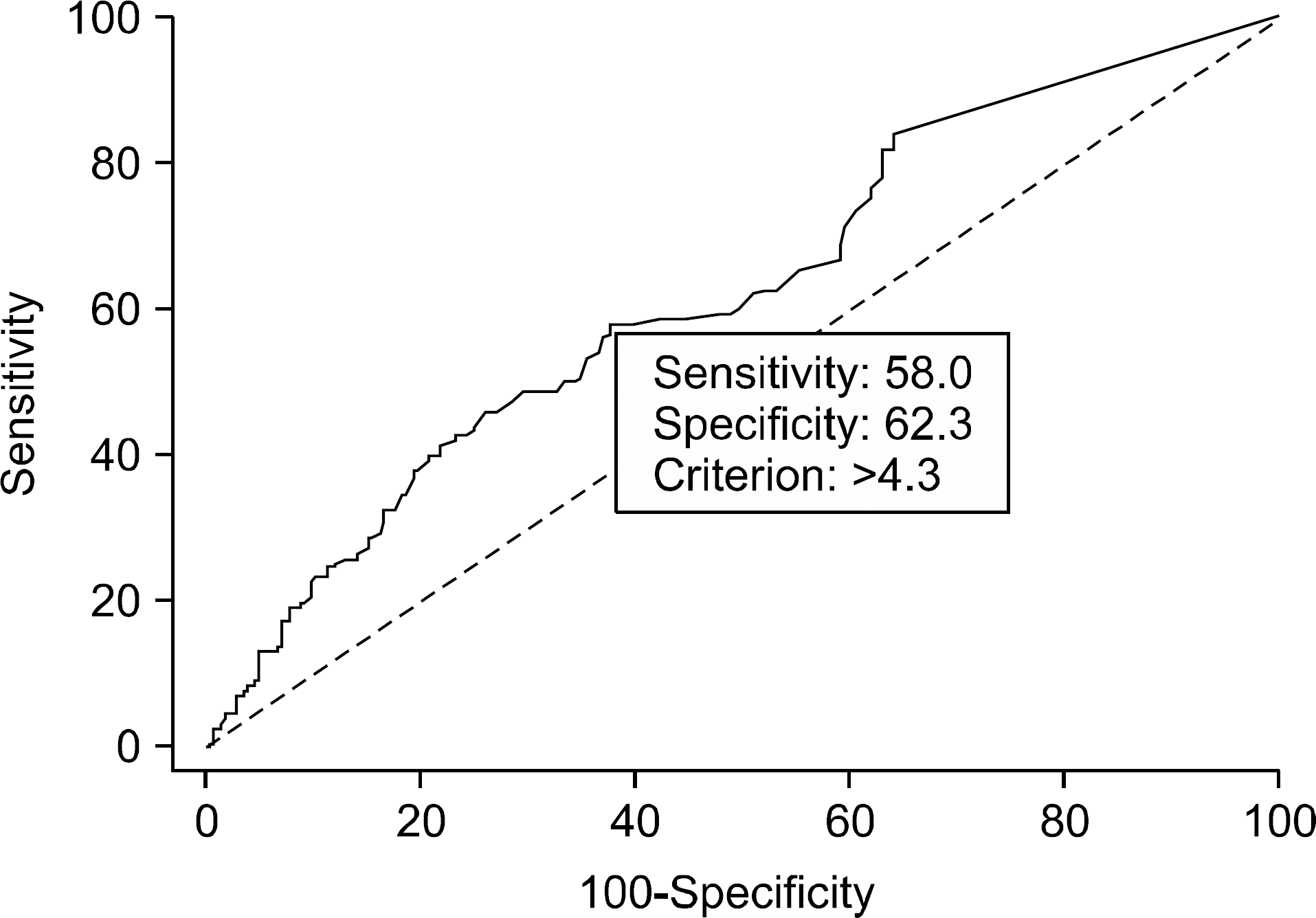
Fig. 2.
Receiver operating characteristic curve of SUVmax. With an SUVmax cutoff value of above 0, the surgeon can predict central lymph node (CLN) metastasis, and proceed total thyroidectomy and CLN dissection.
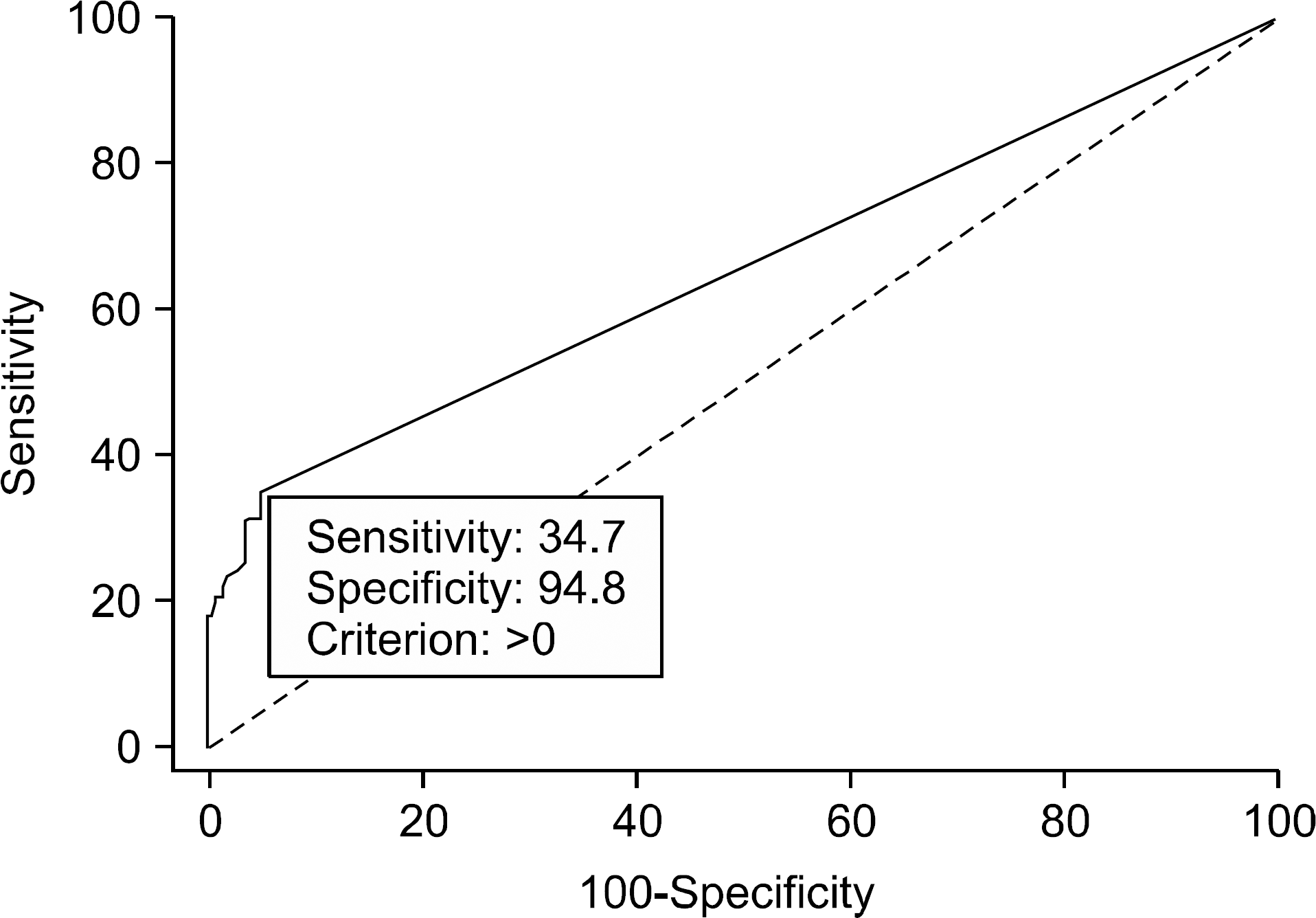
Table 1.
Clinicopathological data in relation to CLN in PTC
Table 2.
Associations of CLN metastasis with AMES, MACIS, SUV
Table 3.
Associations of CLN metastasis with predictive values by multivariate logistic regression analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download