Abstract
for PTC in selected cases. (Korean J Endocrine Surg Purpose: The aim of this study was to evaluate the operative feasibility and safety of endoscopic thyroidectomy via bilateral axillo breast approach (BABA) compared to conventional thyroidectomy in papillary thyroid carcinoma (PTC) patients.
Methods:
From July 2009 to November 2010, patients underwent BABA endoscopic thyroidectomy (ET group; n=41) or conventional open thyroidectomy (OT group; n=61) for PTC. Clinical and pathologic characteristics of patients, operation time, post-operative complications, cosmetic satisfaction and thyroglobulin (TG) level were analyzed retrospectively.
Results:
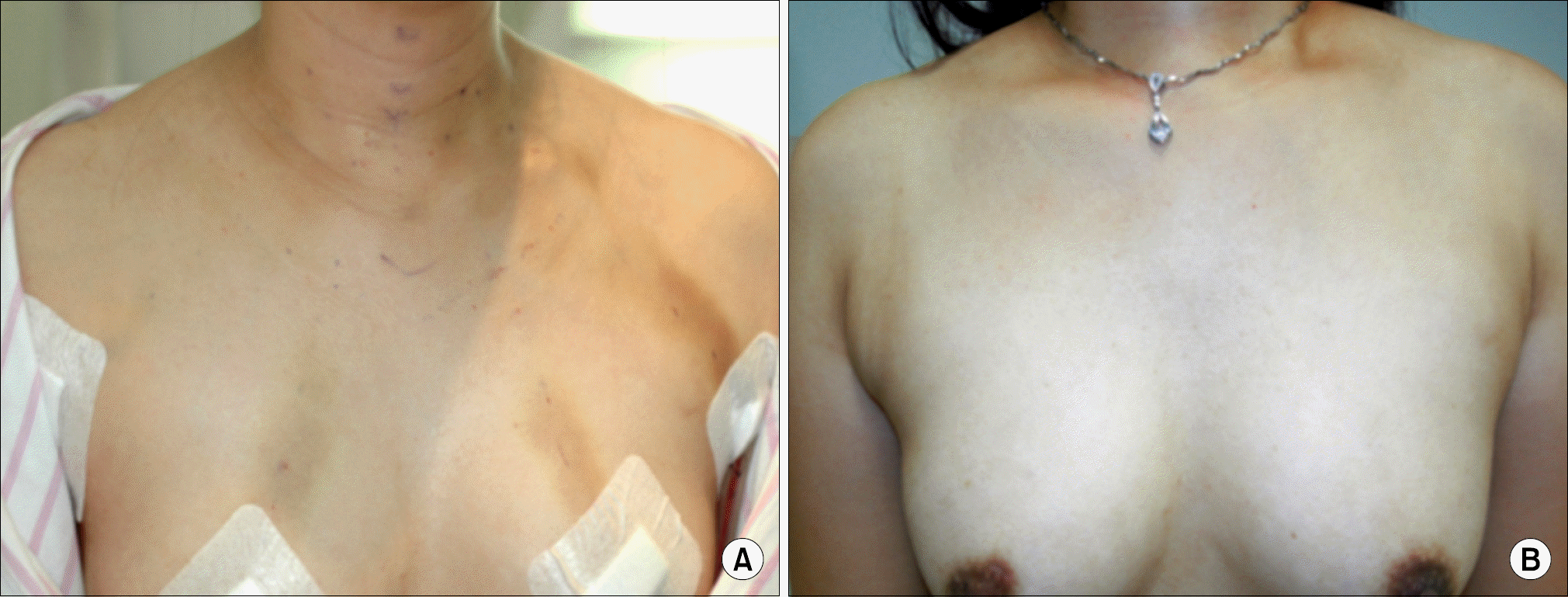
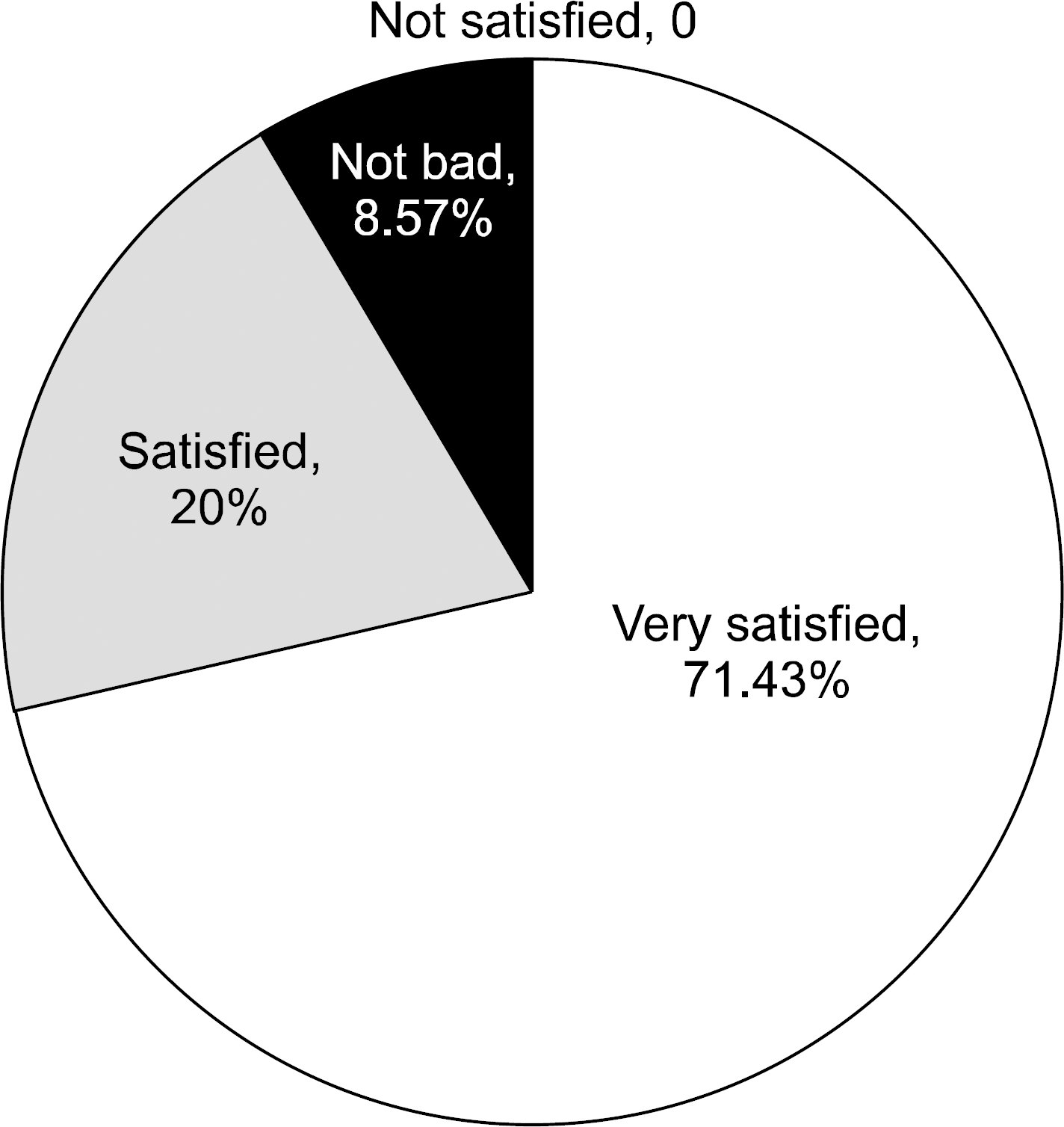
The mean age of the patients was 40.05±9.58 years (range 25∼61 years) and 46.21±13.68 years (range 19∼79 years) for the ET and OT group, respectively. The operative extent in the ET group did not include advanced thyroid cancer or lateral neck dissection. The size of the tumor was 0.78±0.59 cm (range 0.1∼3.00 cm) and 1.54 ±1.05 cm (range 0.3∼6.00 cm) for the ET and OT group, respectively. Extrathyroidal extension and number of retrieved lymph nodes were significantly higher in the OT group. Postoperative radioactive iodine ablation was performed on 25 patients (72.43%) in the ET group and 48 patients (78.69%) in the OT group. There was no abnormal uptake on radioactive iodine scans in the iodine-treated patients and no significant differences in postoperative off-T4 TG levels between the two groups. There were no significant differences in operative time, amount of drainage, postoperative hospitalization period, hypocalcemia, and vocal cord palsy between the two groups. Cosmetic results of ET group were rated as excellent in a 3-month postoperative questionnaire by 25 (72.43%) of 35 patients.
Go to : 
REFERENCES
1.Won YJ., Sung J., Jung KW., Kong HJ., Park S., Shin HR, et al. Nationwide Cancer Incidence in Korea, 2003-2005. Cancer Res Treat. 2009. 41:122–31.

2.Jung KW., Park S., Kong HJ., Won YJ., Lee JY., Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011. 43:1–11.

3.Chung YS., Choe JH., Kang KH., Kim SW., Chung KW., Park KS, et al. Endoscopic thyroidectomy for thyroid malignancies: comparison with conventional open thyroidectomy. World J Surg. 2007. 31:2302–6.

4.Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg. 1996. 83:875.

5.Hüscher CS., Chiodini S., Napolitano C., Recher A. Endoscopic right thyroid lobectomy. Surg Endosc. 1997. 11:877.

6.Shimizu K., Akira S., Jasmi AY., Kitamura Y., Kitagawa W., Akasu H, et al. Video-assisted neck surgery: endoscopic resection of thyroid tumors with a very minimal neck wound. J Am Coll Surg. 1999. 188:697–703.

7.Ikeda Y., Takami H., Sasaki Y., Kan S., Niimi M. Endoscopic resection of thyroid tumors by the axillary approach. J Cardiovasc Surg (Torino). 2000. 41:791–2.
8.Ohgami M., Ishii S., Arisawa Y., Ohmori T., Noga K., Furukawa T, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech. 2000. 10:1–4.

9.Berti P., Materazzi G., Galleri D., Donatini G., Minuto M., Miccoli P. Video-assisted thyroidectomy for Graves' disease: report of a preliminary experience. Surg Endosc. 2004. 18:1208–10.

10.Miccoli P., Berti P., Bendinelli C., Conte M., Fasolini F., Martino E. Minimally invasive video-assisted surgery of the thyroid: a preliminary report. Langenbecks Arch Surg. 2000. 385:261–4.

11.Miccoli P., Elisei R., Materazzi G., Capezzone M., Galleri D., Pacini F, et al. Minimally invasive video-assisted thyroidectomy for papillary carcinoma: a prospective study of its completeness. Surgery. 2002. 132:1070–3.

12.Bellantone R., Lombardi CP., Raffaelli M., Boscherini M., Alesina PF., Princi P. Central neck lymph node removal during minimally invasive video-assisted thyroidectomy for thyroid carcinoma: a feasible and safe procedure. J Laparoendosc Adv Surg Tech A. 2002. 12:181–5.

13.Miccoli P., Pinchera A., Materazzi G., Biagini A., Berti P., Faviana P, et al. Surgical treatment of low- and intermediate-risk papillary thyroid cancer with minimally invasive video-assisted thyroidectomy. J Clin Endocrinol Metab. 2009. 94:1618–22.

14.Miccoli P., Materazzi G., Berti P. Minimally invasive thyroidectomy in the treatment of well differentiated thyroid cancers: indications and limits. Curr Opin Otolaryngol Head Neck Surg. 2010. 18:114–8.

15.Chao TC., Lin JD., Chen MF. Gasless video-assisted total thyroidectomy in the treatment of low risk intrathyroid papillary carcinoma. World J Surg. 2004. 28:876–9.

16.Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007. 57:43–66.

17.Juckett G., Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009. 80:253–60.
18.Yoon JH., Park CH., Chung WY. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surg Laparosc Endosc Percutan Tech. 2006. 16:226–31.

19.Choe JH., Kim SW., Chung KW., Park KS., Han W., Noh DY, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg. 2007. 31:601–6.

20.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS., Doherty GM., Haugen BR., Kloos RT., Lee SL., Mandel SJ, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–214.

21.Cady B. Papillary carcinoma of the thyroid gland: treatment based on risk group definition. Surg Oncol Clin N Am. 1998. 7:633–44.
22.Gemsenjäger E., Perren A., Seifert B., Schüler G., Schweizer I., Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003. 197:182–90.

23.Ito Y., Tomoda C., Uruno T., Takamura Y., Miya A., Kobayashi K, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004. 28:498–501.
24.Henry JF., Gramatica L., Denizot A., Kvachenyuk A., Puccini M., Defechereux T. Morbidity of prophylactic lymph node dissection in the central neck area in patients with papillary thyroid carcinoma. Langenbecks Arch Surg. 1998. 383:167–9.

Go to : 
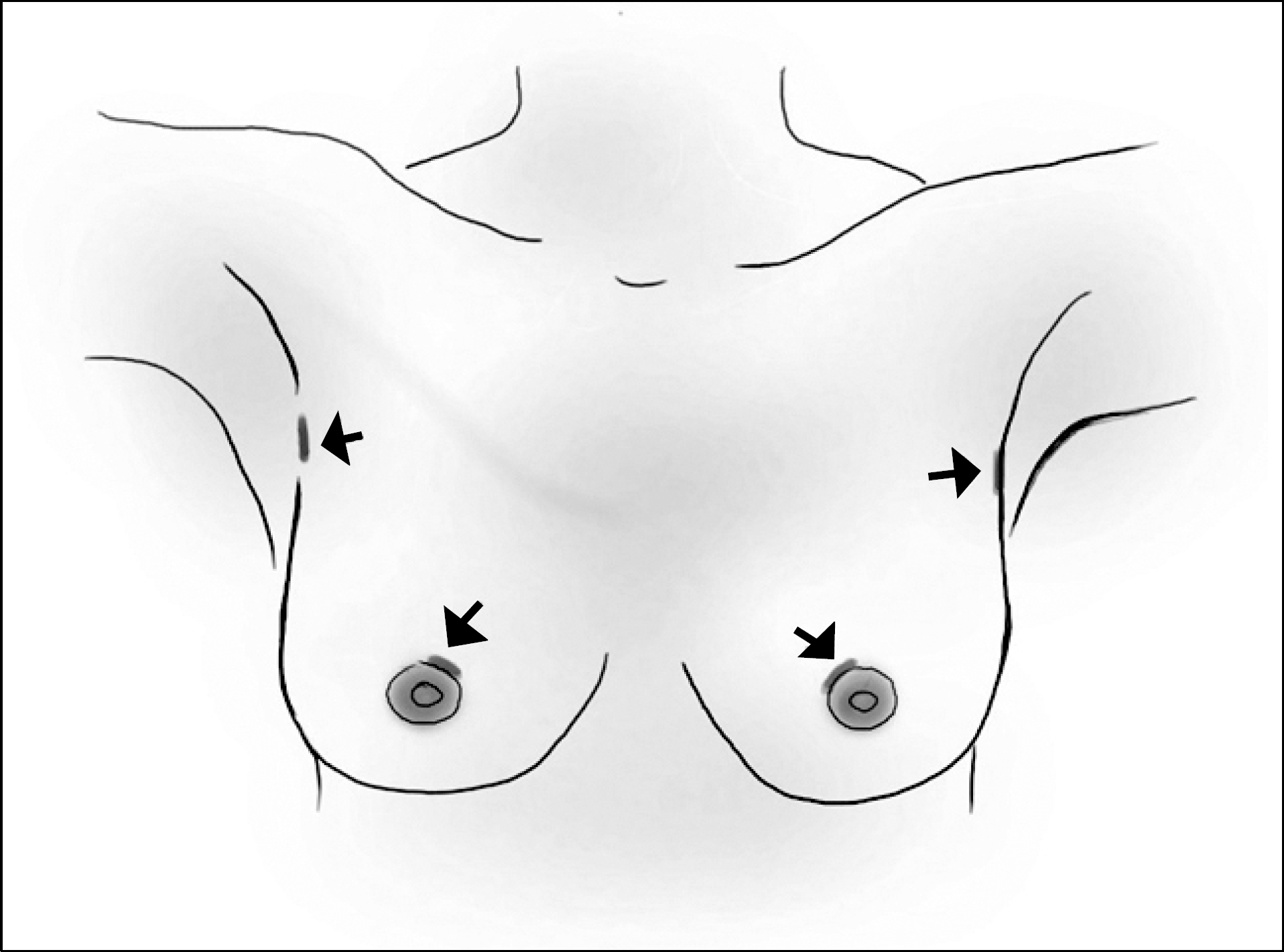 | Fig. 1Location of skin incision in endoscopic thyroidectomy via bilateral axillobreast approach. |
Table 1.
Clinical characteristics of the patients in the two group
Table 2.
Pathologic characteristics and and the results of postoperative RAI ablation of the patients in the two group
| Total | Endoscopic group (n=41) | Open group (n=61) | P value |
|---|---|---|---|
| Tumor size (cm) | 0.78±0.59 (0.1∼3.00) | 1.54±1.05 (0.3∼6.00) | <0.01 |
| Extrathyroidal extension | 11/41 (26.83%) | 35/61 (57.38%) | <0.01 |
| Retrieved lymph node | 5.90±5.64 (1∼20) | 28.48±23.71 (1∼95) | <0.01 |
| [18.91±20.09 (1∼91)]∗ | <0.01 | ||
| Central LN metastasis | 5/41 (12.20%) | 37/61 (60.66%) | <0.01 |
| Lateral LN metastasis | 0/41 (0.00%) | 20/61 (32.79%) | <0.01 |
| Off T4-TG levels† | 0.71±1.69 (0∼8.25) | 1.90±5.73 (0∼41.20) | 0.20 |
| Tg <1.0 (ng/mL)† | 18/25 (72.00%) | 31/48 (64.58%) | 0.31 |
Table 3.
Comparison of postoperative complication between the two group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download