Abstract
The incidence of PTMC (papillary thyroid microcarcinoma) has rapidly increased recently due to the application of ultrasonography to the thyroid. The good prognosis of PTMC is well known with a mortality rate of less than 1%. However, there is controversy about the surgical extent of thyroidectomy for PTMC patients between surgeons and endocrinologists due to differences in understanding the clinical properties of PTMC, while having a difference in basic concepts in the treatment and follow up strategy for PTMC patients. Total thyroidectomy is recommended for PTMC patients because there is no major difference in the rate of lymph node metastasis, extrathyroidal extension, multiplicity between the PTMC and PTC over 1 cm in size and although rare, occasional distant metastasis and mortality cases could be developed. However, there is no evidence of benefit of total thyroidectomy for the survival rate of PTMC patients. The microscopic lymph node metastasis and extrathyroidal extension are not prognostic factors for the survival or recurrence in PTMC. The clinical lateral neck lymph node metastasis and multiplicity has been proposed as valuable prognostic factors in micropapillary carcinoma and these factors could be assessed accurately by ultrasonography preoperatively. A decision on the proper extent of thyroidectomy could be possible in most PTMC patients. This article summarizes available data and concludes that routine total thyroidectomy for PTMC patients is not rational.
REFERENCES
1.Ito Y., Uruno T., Nakano K., Takamura Y., Miya A., Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003. 13:381–7.

2.Pelizzo MR., Boschin IM., Toniato A., Pagetta C., Piotto A., Bernante P, et al. Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun. 2004. 25:547–52.

3.Pelizzo MR., Boschin IM., Toniato A., Piotto A., Bernante P., Pagetta C, et al. Papillary thyroid microcarcinoma (PTMC): prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol. 2006. 32(10):1144–8.

4.Roti E., Rossi R., Trasforini G., Bertelli F., Ambrosio MR., Busutti L, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006. 91:2171–8.

5.Chow SM., Law SC., Chan JK., Au SK., Yau S., Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003. 98:31–40.

6.Van de Velde CJH., Hamming JF., Goslings BM., Schelfhout LJDM., Clark OH., Smeds S, et al. Report of the consensus development conference on the management of differentiated thyroid cancer in the Netherlands. Eur J Cancer Clin Oncol. 1988. 24:287–92.

7.Hay ID., Grant CS., van Heerden JA., Goellner JR., Ebersold JR., Bergstralh EJ. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992. 112:1139–46.
8.Wooner LB., Lemmon ML., Beahrs OH., Black BM., Keating FR Jr. Occult papillary carcinoma of the thyroid gland: a study of 140 cases observed in a 30-year period. J Clin Endocrinol Metab. 1960. 20:89–105.
9.Sampson RJ., Oka H., Key CR., Buncher CR., Iijima S. Metastases from occult thyroid carcinoma. An autopsy study from Hiroshima and Nagasaki, Japan. Cancer. 1970. 25:803–11.
10.Mazzaferri EL., Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981. 70:511–8.
11.Iida F., Sugenoya A., Muramatsu A. Clinical and pathologic properties of small differentiated carcinomas of the thyroid gland. World J Surg. 1991. 15:511–5.
12.Noguchi S., Yamashita H., Uchino S., Watanabe S. Papillary microcarcinoma. World J Surg. 2008. 32:747–53.

13.Hay ID., Hutchinson ME., Gonzalez-Losada T., McIver B., Reinalda ME., Grant CS, et al. Papillary thyroid microcar-cinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008. 144:980–8.

14.Pellegriti G., Scollo C., Lumera G., Regalbuto C., Vigneri R., Belfiore A. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab. 2004. 89:3713–20.

15.Cheema Y., Repplinger D., Elson D., Chen H. Is tumor size the best predictor of outcome for papillary thyroid cancer? Ann Surg Oncol. 2006. 13:1524–8.

16.Furlan JC., Bedard Y., Rosen IB. Biologic basis for the treatment of microscopic, occult well-differentiated thyroid cancer. Surgery. 2001. 130:1050–4.

17.Rosen IB., Azadian A., Walfish PG. Adverse aspects of small thyroid cancer and need for treatment. Head Neck. 1995. 17:373–6.

18.Salvadori B., Del Bo R., Pilotti S., Grassi M., Cusumano F. "Occult" papillary carcinoma of the thyroid: a questionable entity. Eur J Cancer. 1993. 29A:1817–20.

19.Cappelli C., Castellano M., Braga M., Gandossi E., Pirola I., De Martino E, et al. Aggressiveness and outcome of papillary thyroid carcinoma (PTC) versus microcarcinoma (PMC): a mono-institutional experience. J Surg Oncol. 2007. 95:555–60.

20.Noguchi S., Yamashita H., Murakami N., Nakayama I., Toda M., Kawamoto H. Small carcinomas of the thyroid. A long-term follow-up of 867 patients. Arch Surg. 1996. 131:187–91.
21.Rodriguez JM., Moreno A., Parrilla P., Sola J., Soria T., Tebar FJ, et al. Papillary thyroid microcarcinoma: clinical study and prognosis. Eur J Surg. 1997. 163:255–9.
22.Rassael H., Thompson LD., Heffess CS. A rationale for conservative management of microscopic papillary carcinoma of the thyroid gland: a clinicopathologic correlation of 90 cases. Eur Arch Otorhinolaryngol. 1998. 255:462–7.

23.Carcangiu ML., Zampi G., Pupi A., Castagnoli A., Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer. 1985. 55:805–28.

24.Rossi RL., Cady B., Silverman ML., Wool MS., Horner TA. Current results of conservative surgery for differentiated thyroid carcinoma. World J Surg. 1986. 10:612–22.

25.Vickery AL Jr., Wang CA., Walker AM. Treatment of intrathy-roidal papillary carcinoma of the thyroid. Cancer. 1987. 60:2587–95.

26.DeGroot LJ., Kaplan EL., McCormick M., Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990. 71:414–24.

27.Fujimoto Y., Sugitani I. Postoperative prognosis of intrathy-roidal papillary thyroid carcinoma: long-term (35-45 year) follow-up study. Endocr J. 1998. 45:475–84.
28.Sugitani I., Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J. 1999. 46:209–16.

29.Lin JD., Chen ST., Chao TC., Hsueh C., Weng HF. Diagnosis and therapeutic strategy for papillary thyroid microcarcinoma. Arch Surg. 2005. 140:940–5.

30.Sugitani I., Yanagisawa A., Shimizu A., Kato M., Fujimoto Y. Clinicopathologic and immunohistochemical studies of papillary thyroid microcarcinoma presenting with cervical lymphadenopathy. World J Surg. 1998. 22:731–7.

31.Baudin E., Travagli JP., Ropers J., Mancusi F., Bruno-Bossio G., Caillou B, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998. 83:553–9.
32.Yamashita H., Noguchi S., Murakami N., Toda M., Yamashita H., Uchino S, et al. Extracapsular invasion of lymph node metastasis. A good indicator of disease recurrence and poor prognosis in patients with thyroid microcarcinoma. Cancer. 1999. 86:842–9.
33.Kim TY., Hong SJ., Kim JM., Gu Kim W., Gong G., Ryu JS, et al. Prognostic parameters for recurrence of papillary thyroid microcarcinoma. BMC Cancer. 2008. 8:296–307.

34.Ito Y., Tomoda C., Uruno T., Takamura Y., Miya A., Kobayashi K, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004. 28:498–501.
35.Ito Y., Tomoda C., Uruno T., Takamura Y., Miya A., Kobayashi K, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006. 30:91–9.

36.Ito Y., Tomoda C., Uruno T., Takamura Y., Miya A., Kobayashi K, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg. 2006. 30:780–6.

37.Ito Y., Tomoda C., Uruno T., Takamura Y., Miya A., Kobayashi K, et al. Minimal extrathyroid extension does not affect the relapse-free survival of patients with papillary thyroid carcinoma measuring 4 cm or less over the age of 45 years. Surg Today. 2006. 36:12–8.
38.Ito Y., Miyauchi A., Jikuzono T., Higashiyama T., Takamura Y., Miya A, et al. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg. 2007. 31:838–48.

39.Sanders LE., Rossi RL. Occult well differentiated thyroid carcinoma presenting as cervical node disease. World J Surg. 1995. 19:642–7.

40.Sugino K., Kure Y., Iwasaki H., Ozaki O., Mimura T., Matsumoto A, et al. Metastases to the regional lymph nodes, lymph node recurrence, and distant metastases in nonadvanced papillary thyroid carcinoma. Surg Today. 1995. 25:324–8.

41.Hay ID. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol. 2006. 94:692–700.

42.Sawka AM., Thephamongkhol K., Brouwers M., Thabane L., Browman G., Gerstein HC. Clinical review 170: a systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004. 89:3668–76.
43.Pacini F., Schlumberger M., Harmer C., Berg GG., Cohen O., Duntas L, et al. Post-surgical use of radioiodine (131I) in patients with papillary and follicular thyroid cancer and the issue of remnant ablation: a consensus report. Eur J Endo-crinol. 2005. 153:651–9.

44.Cailleux AF., Baudin E., Travagli JP., Ricard M., Schlumberger M. Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? J Clin Endocrinol Metab. 2000. 85:175–8.

45.Torlontano M., Attard M., Crocetti U., Tumino S., Bruno R., Costante G, et al. Follow-up of low risk patients with papillary thyroid cancer: Role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab. 2004. 89:3402–7.

46.Torlontano M., Crocetti U., Augello G., D'Aloiso L., Bonfitto N., Varraso A, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endo-crinol Metab. 2006. 91:60–3.
47.Schlumberger M., Berg G., Cohen O., Duntas L., Jamar F., Jarzab B, et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol. 2004. 150:105–12.

48.Kim TY., Kim WB., Kim ES., Ryu JS., Yeo JS., Kim SC, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005. 90:1440–5.
49.Grossman RF., Tu SH., Duh QY., Siperstein AE., Novosolov F., Clark OH. Familial nonmedullary thyroid cancer. An emerging entity that warrants aggressive treatment. Arch Surg. 1995. 130:892–9.

50.Alsanea O., Wada N., Ain K., Wong M., Taylor K., Ituarte PH, et al. Is familial non-medullary thyroid carcinoma more aggressive than sporadic thyroid cancer? A multicenter series. Surgery. 2000. 128:1043–51.

52.Triponez F., Wong M., Sturgeon C., Caron N., Ginzinger DG., Segal MR, et al. Does familial non-medullary thyroid cancer adversely affect survival? World J Surg. 2006. 30:787–93.

53.Sippel RS., Caron NR., Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: Screening, clinical management, and follow-up. World J Surg. 2007. 31:924–33.

54.Uchino S., Noguchi S., Kawamoto H., Yamashita H., Watanabe S., Yamashita H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002. 26:897–902.

55.Ito Y., Kakudo K., Hirokawa M., Fukushima M., Yabuta T., Tomoda C, et al. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009. 145:100–5.

56.Maxwell EL., Hall FT., Freeman JL. Familial non-medullary thyroid cancer: a matched-case control study. Laryngoscope. 2004. 114:2182–6.

57.Loh KC. Familial nonmedullary thyroid carcinoma: a meta-review of case series. Thyroid. 1997. 7:107–13.

58.Piper HG., Bugis SP., Wilkins GE., Walker BA., Wiseman S., Baliski CR. Detecting and defining hypothyroidism after hemithyroidectomy. Am J Surg. 2005. 189:587–91.

59.Miller FR., Paulson D., Prihoda TJ., Otto RA. Risk factors for the development of hypothyroidism after hemithyroidectomy. Arch Otolaryngol Head Neck Surg. 2006. 132:36–8.

60.De Carlucci D Jr., Tavares MR., Obara MT., Martins LA., Hojaij FC., Cernea CR. Thyroid function after unilateral total lobectomy: risk factors for postoperative hypothyroidism. Arch Otolaryngol Head Neck Surg. 2008. 134:1076–9.
61.Antonelli A., Miccoli P., Ferdeghini M., Di Coscio G., Alberti B., Iacconi P, et al. Role of neck ultrasonography in the follow-up of patients operated on for thyroid cancer. Thyroid. 1995. 5:25–8.

Table 1.
Results of surgical treatment for PTMC patients at Noguchi Clinic and Mayo Clinic
Table 2.
Cervical lymph node recurrence according to central lymph node metastasis
Fig. 1
Lymph node recurrence free survival of the papillary thyroid cancer patients with central L-N metastasis and without central L-N metastasis.
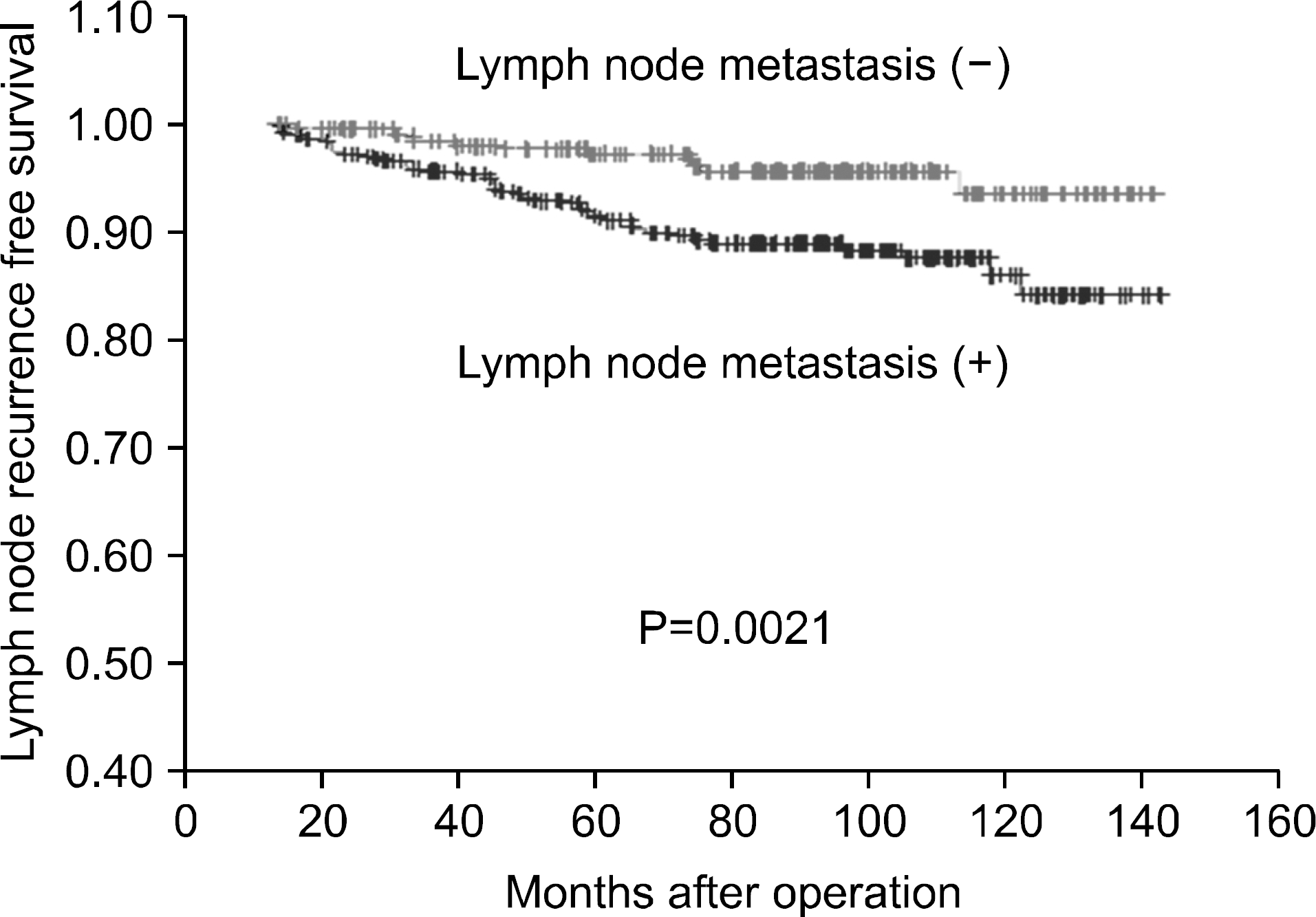
Fig. 2
Lymph node recurrence free survival of the papillary thyroid cancer patients with microscopic central L-N metastasis and clinical central L-N metastasis.
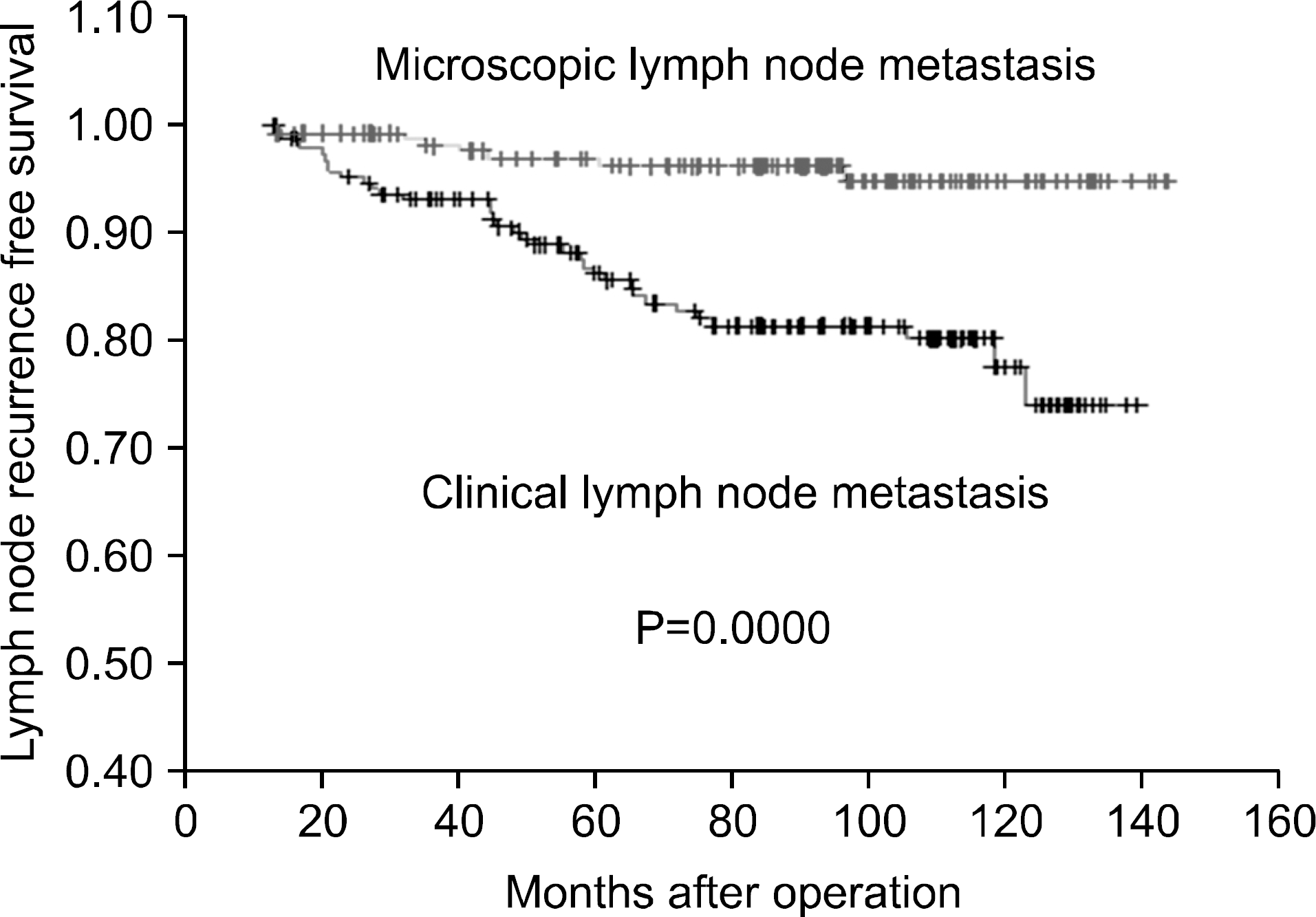
Fig. 3
Lymph node recurrence free survival of the papillary thyroid cancer patients with microscopic central L-N metastasis and without central L-N metastasis.
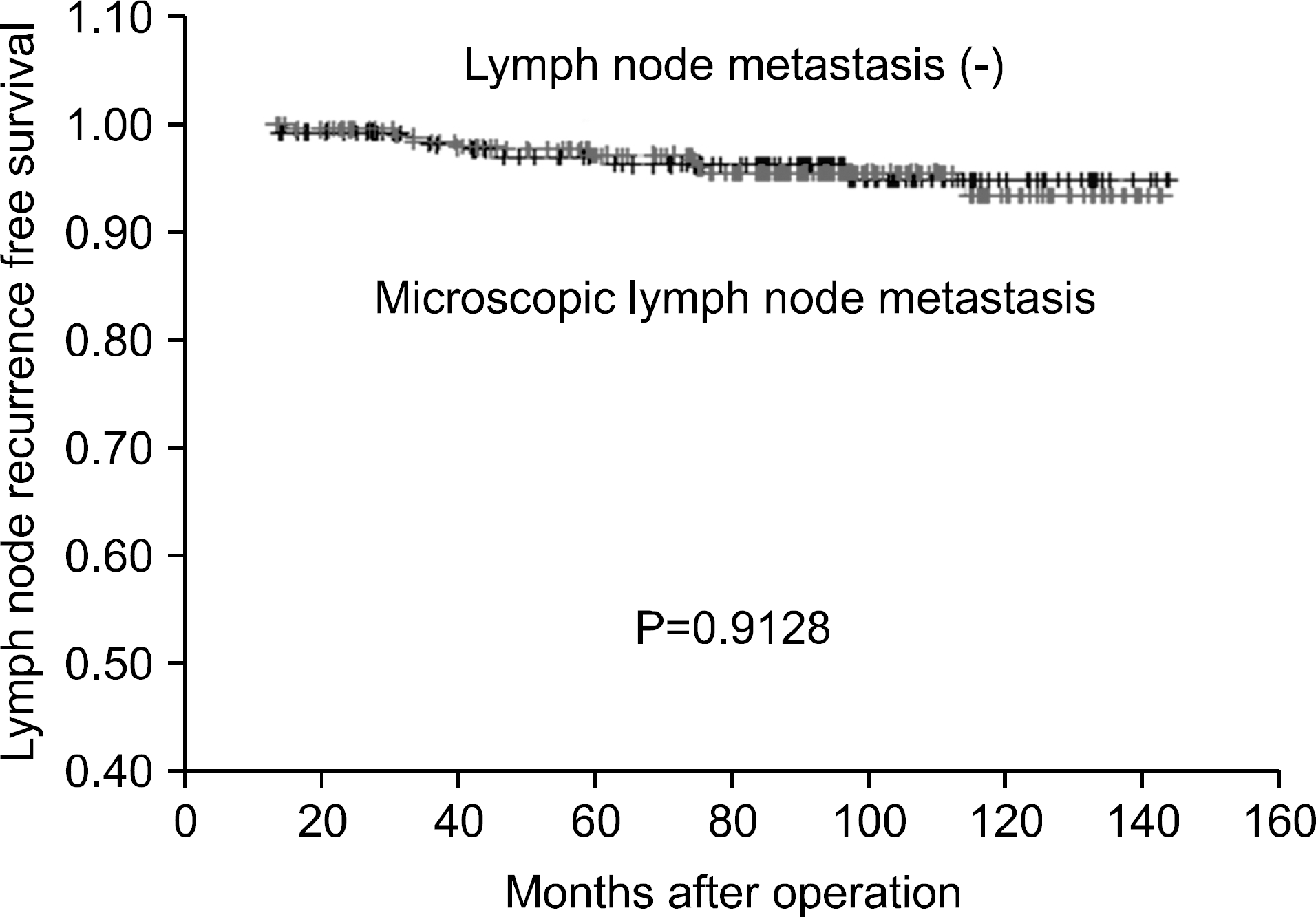
Fig. 5
Disease free survival of PTMC patients with various extrathyroidal extension by gross findings.
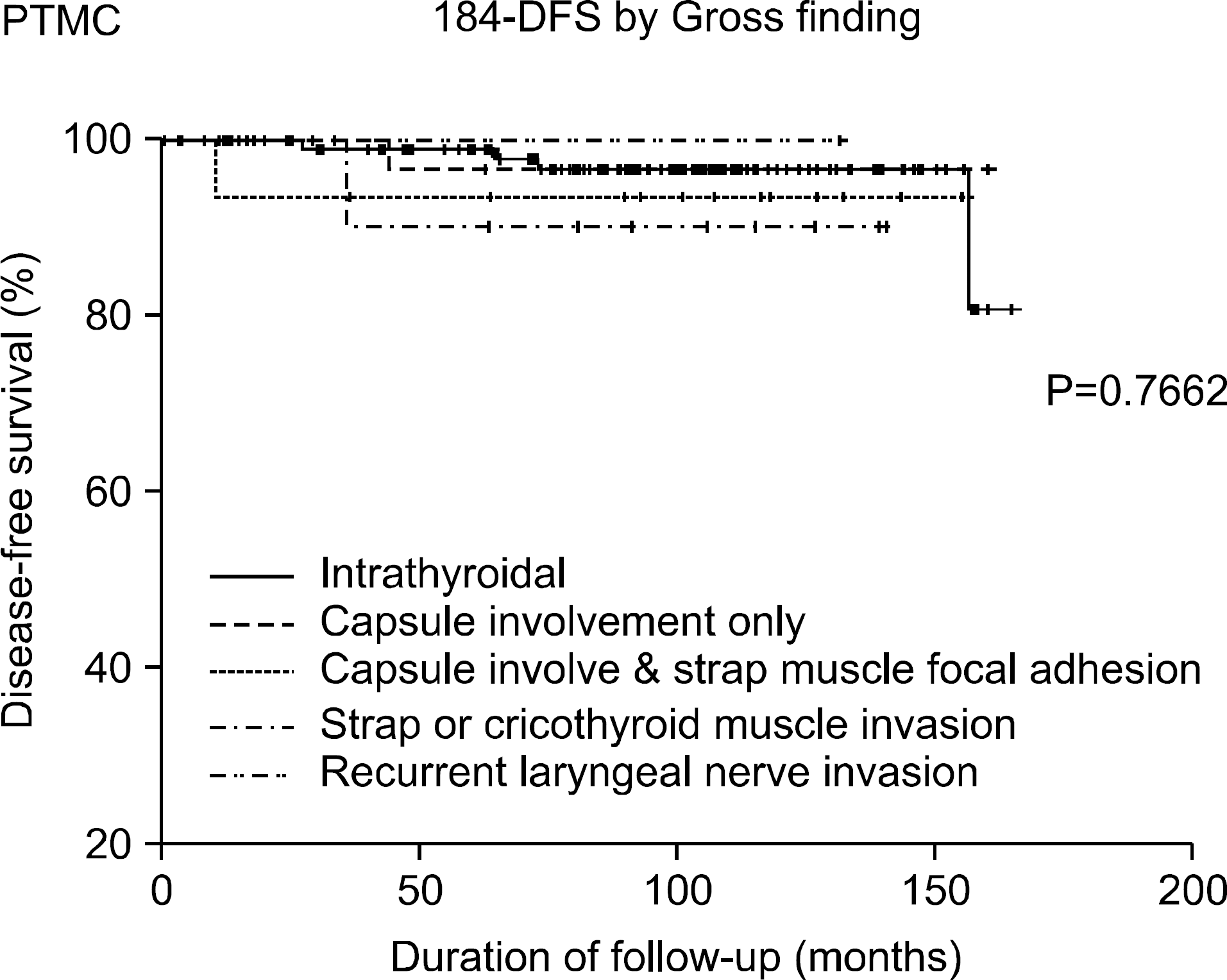
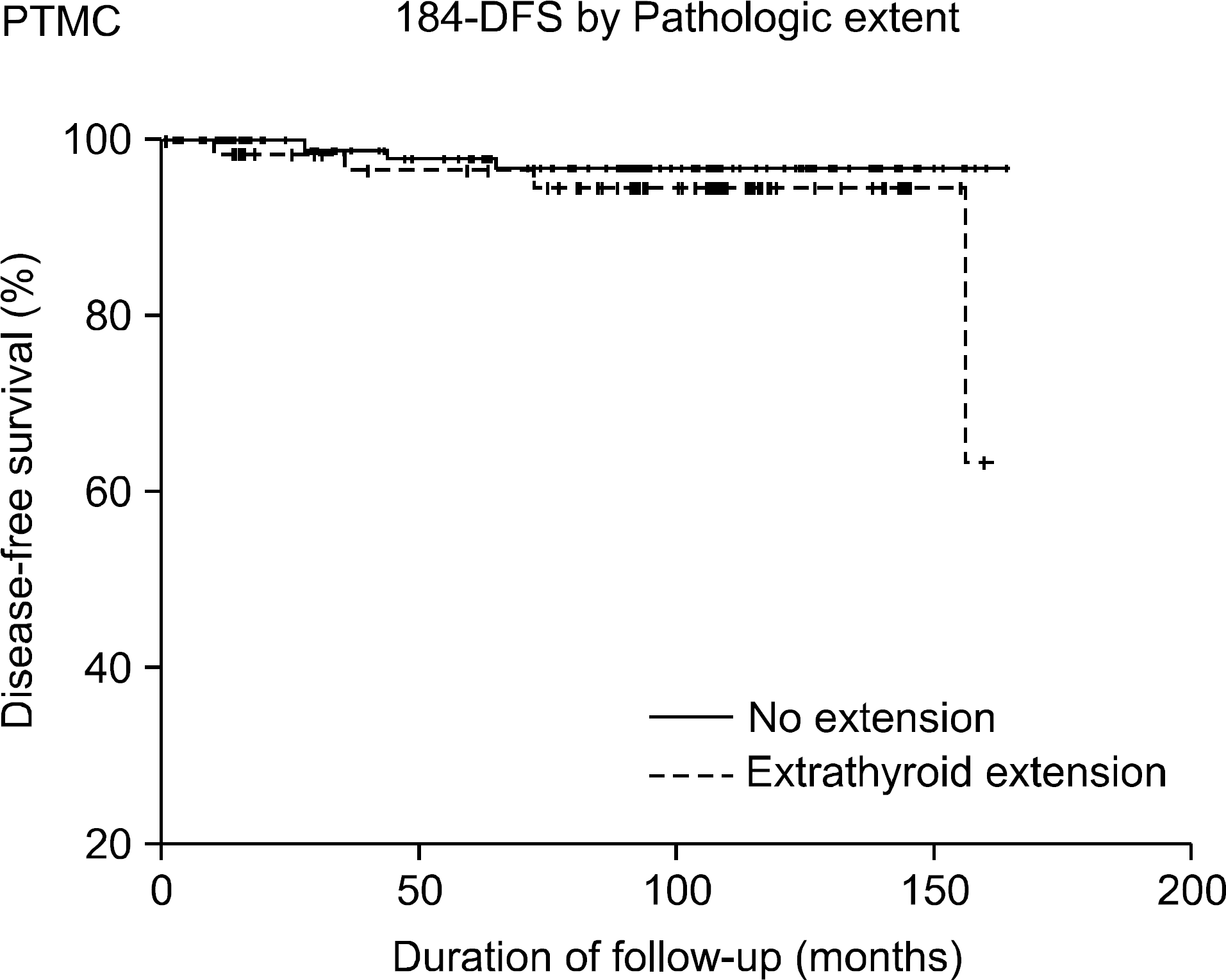
Fig. 6
Disease free survival of PTMC patients without extrathyroidal extension by gross finding but pathdogially extrathyroidal extension positive and pathologically extrathyroidal extension negative.
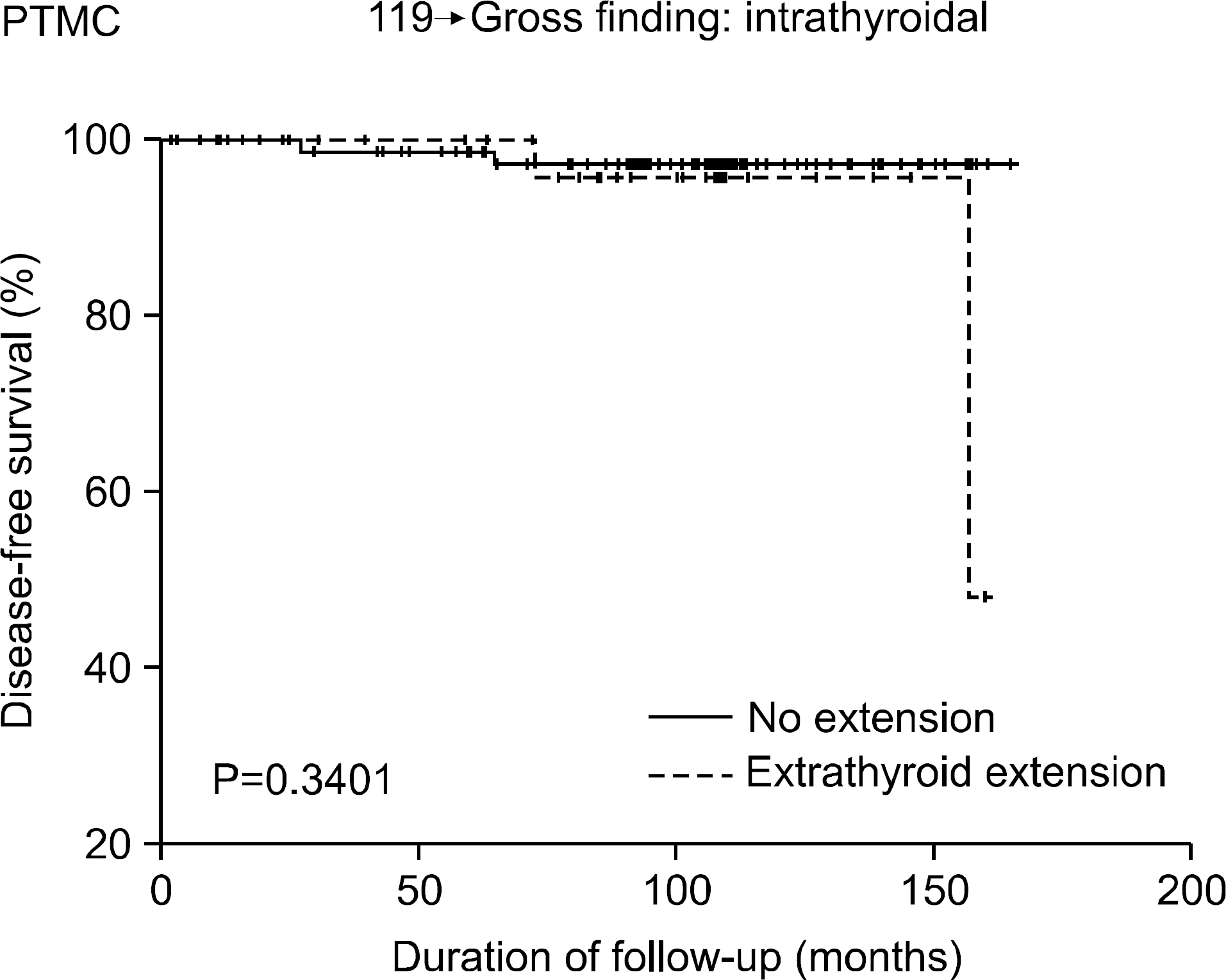
Table 3.
Multifocality in PTMC patients
| Author | Frequency (%) |
|---|---|
| Baudin | 60∗ |
| Furlan | 31.9 |
| Chow | 31 |
| Pellegriti | 26.7 |
| Salvadori | 23.6 |
| Rassael | 23 |
| Lin | 19 |
Table 4.
Recurrence in remnant thyroid following lobectomy for PTMC patients
| Author | Frequency (%) |
|---|---|
| Hay | 10 |
| Baudin | 3.5 |
| 1.7 unifocal PTMC | |
| 8.0 multifocal PTMC | |
| Noguchi | 0.6 |
| Rassael | 0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download