Abstract
Purpose:
Prognosis of papillary thyroid carcinoma (PTC) is very favorable, but PTC frequently invade adjacent soft tissue and metastases to cervical lymph node. We evaluated the pattern of cervical neck lymph node metastasis in PTC according to tumor size.
Methods:
From August 2005 to January 2009, 353 patients were underwent surgery for PTC. Among these patients, total thyroidectomy with cervical neck lymph node dissection were done in 266 patients. We subdivided patients into four groups according to size and compared the clinicopathologic characters between groups. And we confirmed the factors affecting central neck node metastasis.
Results:
The mean age of patients of diagnosis was 49.1 years and female to male ratio was 5.8:1. Cervical lymph node metastasis were in 47.0% of the total cases. Cervical lymph node metastases and invasion to adjacent structure increased with tumor size. But, there were no significant differences in tumor size, invasion to adjacent structure, multifocality or bilaterality according to cervical lymph node metastasis. Early diagnostic age and sexuality were significantly related to cervical lymph node metastasis of PTC.
Conclusion:
PTC showed the aggressiveness with increasing tumor size. Tumor size was not related to cervical lymph node metastasis. These findings suggest that tumor size can help treat PTC, can't be used by prediction factor of cervical lymph node metastasis. (Korean J Endocrine Surg 2010;10:256-260)
Go to : 
REFERENCES
1.Davies L., Welch HG. Increasing incidence of thyroid cancer in the United states, 1973-2002. JAMA. 2006. 295:2164–7.

2.Fahey TJ 3rd., Reeve TS., Delbridge L. Increasing incidence and changing presentation of thyroid cancer over a 30-year period. Br J Surg. 1995. 82:518–20.

3.Liu S., Seminensiw R., Ugnat AM., Mao Y. Increasing thyroid cancer in Canada, 1970-1996: time trends and age-period-cohort effects. Br J Cancer. 2001. 85:1335–9.
4.Hodgson NC., Button J., Solorzano CC. Thyroid cancer: Is the incidence still increasing? Ann Surg Oncol. 2004. 11:1093–7.

5.보건복지가족부, 중앙암등록본부. 국가등록사업 연례 보 고서(2005년 암발생, 1993-2005 암생존현황). 서울, 한국: 보건복지가족부;2008. p. 18–91.
6.Sherman SI. Thyroid carcinoma. Lancet. 2003. 361:501–11.
7.Mazzaferri EL., Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994. 97:418–28.

8.Cooper DS., Doherty GM., Haugen BR., Kloos RT., Lee SL., Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid . 2006. 16:109–42.

9.Pacini F., Schlumberger M., Dralle H., Elisei R., Smit JW., Wiersinga W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006. 154:787–803.

10.Schlumberger M., Pacini F. Local anc regional recurrences. In: Schlumberger M, Pacini F editors. Thyroid Tumors. 2nd ed.Paris: Editions Nucleon;2003. p. 181–92.
11.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002. 87:13–5.
12.Byar DP., Green SB., Dor P., Williams ED., Colon J., van Gilse HA, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979. 15:1033–41.

13.Cady B., Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988. 104:947–53.
14.DeGroot LJ., Kaplan EL., McCormick M., Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990. 71:414–24.

15.Hay ID., Bergstralh EJ., Goellner JR., Ebersold JR., Grant CS. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993. 114:1050–7.
16.Mazzaferri EL., Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994. 97:418–28.

17.Sherman SI., Brierley JD., Sperling M., Ain KB., Bigos ST., Cooper DS, et al. Prospective multicenter study of thyroid carcinoma treatment: Initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998. 83:1012–21.
18.Mazzaferri EL., Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001. 86:1447–63.
19.Mazzaferri EL., Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994. 97:418–28.

20.Tsang RW., Brierley JD., Simpson WJ., Panzarella T., Gospodarowicz MK., Sutcliffe SB. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998. 82:375–88.

21.Yamashita H., Noguchi S., Murakami N., Kawamoto H., Watanabe S. Extracapsular invasion of lymph node metastasis is an indicator of distant metastasis and poor prognosis in patients with thyroid papillary carcinoma. Cancer. 1997. 80:2268–72.

22.Pacini F., Elisei R., Capezzone M., Miccoli P., Molinaro E., Basolo F, et al. Contralateral papillary thyroid cancer is frequent at completion thyroidectomy with no difference in low- and high-risk patients. Thyroid. 2001. 11:877–81.

23.Shaha AR., Shah JP., Loree TR. Low-risk differentiated thyroid cancer: the need for selective treatment. Ann Surg Oncol. 1997. 4:328–33.

24.Son GM., Han KT. Multicentricity of papillary thyroid carcinoma in contralateral lobe: a comparison of preoperative ultrasonographic findings with those of postoperative histopathologic examination. J Korean Surg Soc. 2003. 65:389–96.
25.Bramley MD., Harrison BJ. Papillary microcarcinoma of thyroid gland. Br J Surg. 1996. 83:1674–83.
26.Lee YM., Bae JW., Son GS. The clinical significance of minimal extrathyroid extension in patients with papillary thyroid microcarcinoma. Korean J Endocrine Surg. 2008. 8:243–9.

27.Park WC., Jung SS., Jeun HM., Kim JS., Jeon HM. The pattern of cervical lymph node metastases in papillary thyroid cancer. Korean J Endocrine Surg. 2007. 7:94–7.
Go to : 
Table 1.
Comparison of clinicopathologic characteristics between four groups
Table 2.
Univariate analysis of clinicopathological characteristic in central neck node metastasis
Table 3.
Multiivariate analysis of clinicopathological character istics in central neck node metastasis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


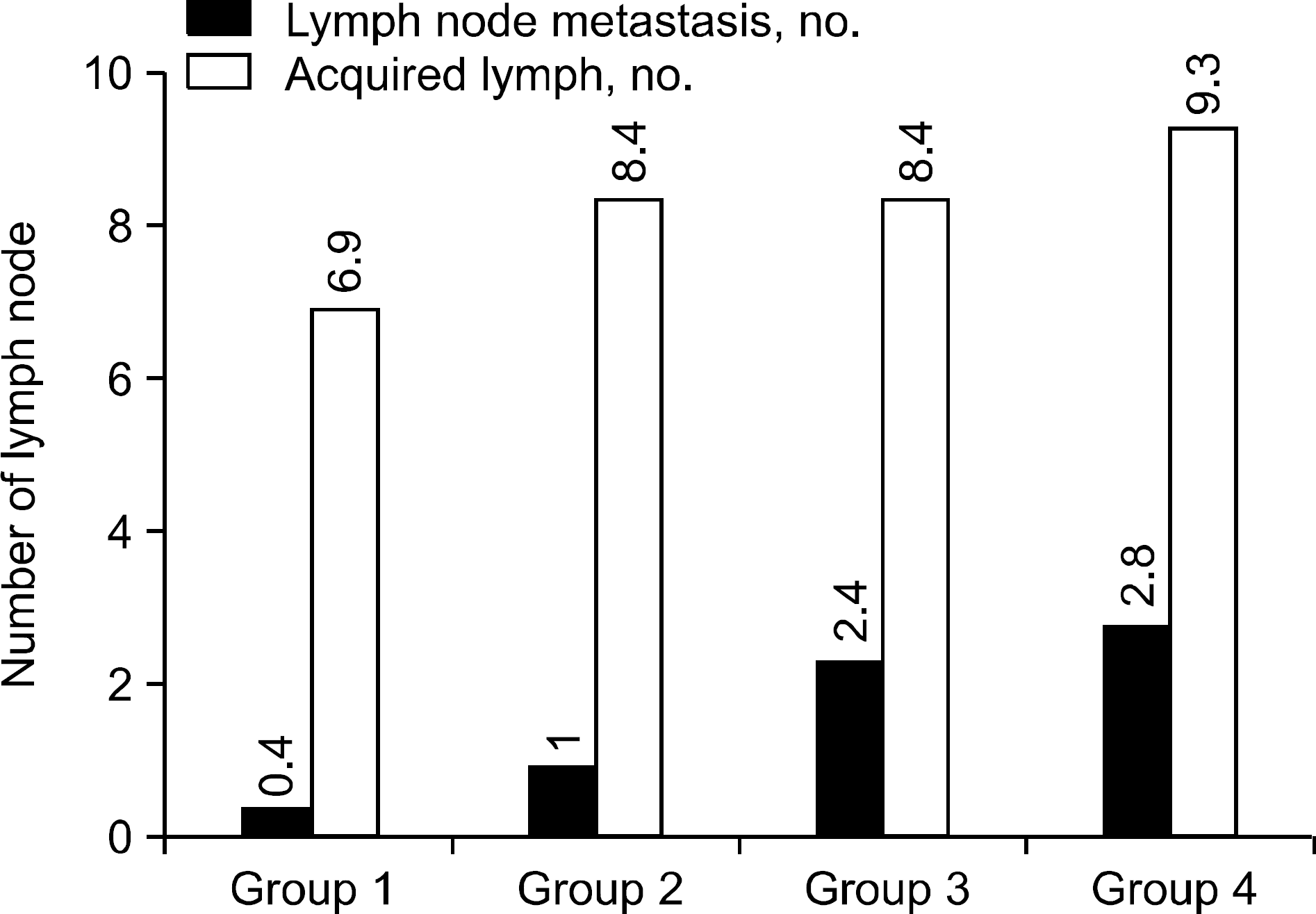
 XML Download
XML Download