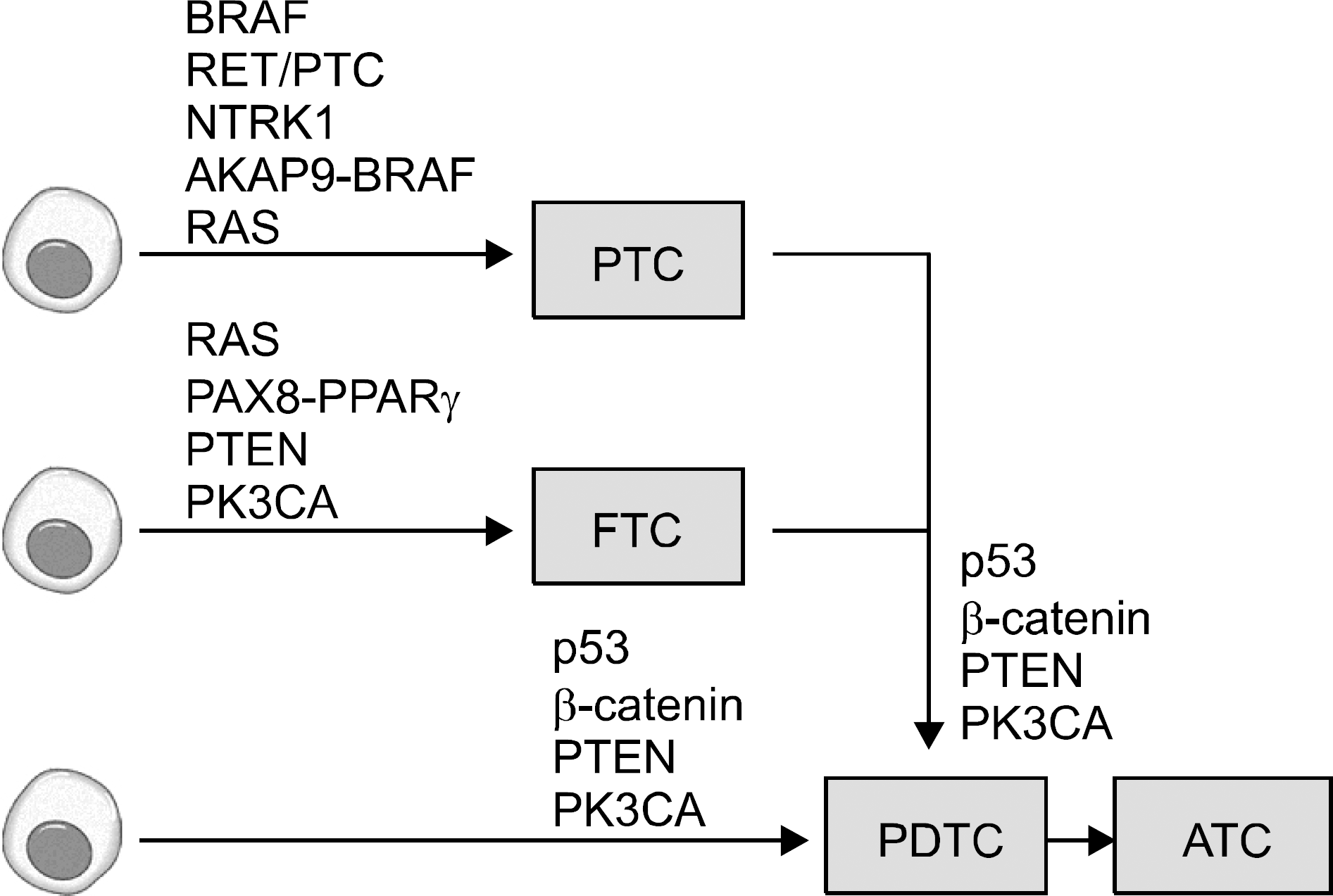
Abstract
The molecular approaches to human diseases are receiving greater attention following the completion of the Human Genome Project. Molecular biology techniques are being widely applied to the field of tumor biology, and thyroid carcinomas are not an exception; several genetic alterations have been suggested to play roles in thyroid carcinogenesis and its progression. Malignant tumors arising from thyroid follicular cells can be classified into papillary carcinoma, follicular carcinoma, poorly differentiated carcinoma and anaplastic carcinoma. BRAF mutation, RET/PTC rearrangement and RAS mutation are the suggested molecular causes of papillary thyroid carcinoma (PTC). RAS mutation, PAX8-PPARγrearrangement, PTEN mutation or methylation, and PIK3CA mutation are known to induce follicular thyroid carcinoma (FTC). Poorly differentiated thyroid carcinoma (PDTC) and anaplastic thyroid carcinoma (ATC) are related to adding p53 or β-catenin gene alterations to those of papillary or follicular carcinomas. The more aggressive genetic alterations are added stepwise as thyroid tumors advance from differentiated PTC or FTC to less differentiated PDTC and finally to ATC. Studying the molecular mechanisms underlying thyroid carcinogenesis may help overcome the limitations of the current diagnostic methods and this may provide more accurate diagnostic and prognostic tools. Furthermore, research at the molecular level is essential for personalized therapies and creating targeted therapies for thyroid carcinomas.
REFERENCES
1.Burman KD., Ringel MD., Wartofsky L. Unusual types of thyroid neoplasms. Endocrinol Metab Clin North Am. 1996. 25:49–68.

2.Riesco-Eizaguirre G., Santisteban P. New insights in thyroid follicular cell biology and its impact in thyroid cancer therapy. Endocr Relat Cancer. 2007. 14:957–77.

3.Wreesmann V., Singh B. Clinical impact of molecular analysis on thyroid cancer management. Surgical Oncology Clinics of North America. 2008. 17:1–35.

4.El-Shabrawi Y., Mangge H., Hermann J. Anti-tumour necrosis factor alpha treatment in chronic recurrent inflammation of the anterior segment of the eye in patients resistant to standard immunomodulatory treatment. Ann Rheum Dis. 2003. 62:1243–4.
5.Roque L., Rodrigues R., Pinto A., Moura-Nunes V., Soares J. Chromosome imbalances in thyroid follicular neoplasms: a comparison between follicular adenomas and carcinomas. Genes Chromosomes Cancer. 2003. 36:292–302.

6.Kitamura Y., Shimizu K., Ito K., Tanaka S., Emi M. Allelotyping of follicular thyroid carcinoma: frequent allelic losses in chromosome arms 7q, 11p, and 22q. J Clin Endocrinol Metab. 2001. 86:4268–72.

7.Castro P., Eknaes M., Teixeira MR., Danielsen HE., Soares P., Lothe RA, et al. Adenomas and follicular carcinomas of the thyroid display two major patterns of chromosomal changes. J Pathol. 2005. 206:305–11.

8.Wreesmann VB., Ghossein RA., Hezel M., Banerjee D., Shaha AR., Tuttle RM, et al. Follicular variant of papillary thyroid carcinoma: genome-wide appraisal of a controversial entity. Genes Chromosomes Cancer. 2004. 40:355–64.

9.Roque L., Nunes VM., Ribeiro C., Martins C., Soares J. Karyotypic characterization of papillary thyroid carcinomas. Cancer. 2001. 92:2529–38.

10.Singh B., Lim D., Cigudosa JC., Ghossein R., Shaha AR., Poluri A, et al. Screening for genetic aberrations in papillary thyroid cancer by using comparative genomic hybridization. Surgery. 2000. 128:888–93. discussion 93-4.

11.Kjellman P., Lagercrantz S., Hoog A., Wallin G., Larsson C., Zedenius J. Gain of 1q and loss of 9q21.3-q32 are associated with a less favorable prognosis in papillary thyroid carcinoma. Genes Chromosomes Cancer. 2001. 32:43–9.

12.Roque L., Soares J., Castedo S. Cytogenetic and fluorescence in situ hybridization studies in a case of anaplastic thyroid carcinoma. Cancer Genet Cytogenet. 1998. 103:7–10.

13.Mark J., Ekedahl C., Dahlenfors R., Westermark B. Cytogenetical observations in five human anaplastic thyroid carcinomas. Hereditas. 1987. 107:163–74.

14.Jenkins RB., Hay ID., Herath JF., Schultz CG., Spurbeck JL., Grant CS, et al. Frequent occurrence of cytogenetic abnormalities in sporadic nonmedullary thyroid carcinoma. Cancer. 1990. 66:1213–20.

15.Rodrigues RF., Roque L., Krug T., Leite V. Poorly differentiated and anaplastic thyroid carcinomas: chromosomal and oligo-array profile of five new cell lines. Br J Cancer. 2007. 96:1237–45.

16.Smallridge RC., Marlow LA., Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009. 16:17–44.

17.Rapp UR., Goldsborough MD., Mark GE., Bonner TI., Groffen J., Reynolds FH Jr, et al. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983. 80:4218–22.

18.Nucera C., Goldfarb M., Hodin R., Parangi S. Role of B-Raf (V600E) in differentiated thyroid cancer and preclinical validation of compounds against B-Raf (V600E). Biochim Biophys Acta. 2009. 1795:152–61.
19.Leicht DT., Balan V., Kaplun A., Singh-Gupta V., Kaplun L., Dobson M, et al. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007. 1773:1196–212.

20.Emuss V., Garnett M., Mason C., Marais R. Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res. 2005. 65:9719–26.

21.Davies H., Bignell GR., Cox C., Stephens P., Edkins S., Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002. 417:949–54.

22.Kumar R., Angelini S., Czene K., Sauroja I., Hahka-Kemppinen M., Pyrhonen S, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003. 9:3362–8.
23.Nikiforova MN., Stringer JR., Blough R., Medvedovic M., Fagin JA., Nikiforov YE. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science. 2000. 290:138–41.

24.Wan PT., Garnett MJ., Roe SM. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004. 116:855–67.

25.Nikiforova MN., Kimura ET., Gandhi M., Biddinger PW., Knauf JA., Basolo F, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003. 88:5399–404.

27.Chung KW., Yang SK., Lee GK., Kim EY., Kwon S., Lee SH, et al. Detection of BRAFV600E mutation on fine needle aspiration specimens of thyroid nodule refines cyto-pathology diagnosis, especially in BRAF600E mutation-prevalent area. Clin Endocrinol (Oxf). 2006. 65:660–6.
28.Jo YS., Li S., Song JH., Kwon KH., Lee JC., Rha SY, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006. 91:3667–70.

29.Xing M., Vasko V., Tallini G., Larin A., Wu G., Udelsman R, et al. BRAF T1796A transversion mutation in various thyroid neoplasms. J Clin Endocrinol Metab. 2004. 89:1365–8.

30.Xing M., Westra WH., Tufano RP., Cohen Y., Rosenbaum E., Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005. 90:6373–9.

31.Kim TY., Kim WB., Rhee YS., Song JY., Kim JM., Gong G, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2006. 65:364–8.

32.Riesco-Eizaguirre G., Gutierrez-Martinez P., Garcia-Cabezas MA., Nistal M., Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I-targeting to the membrane. Endocr Relat Cancer. 2006. 13:257–69.
33.Fecher LA., Cummings SD., Keefe MJ., Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007. 25:1606–20.

34.Ugolini C., Giannini R., Lupi C., Salvatore G., Miccoli P., Proietti A, et al. Presence of BRAF V600E in very early stages of papillary thyroid carcinoma. Thyroid. 2007. 17:381–8.

35.Kim TY., Kim WB., Song JY., Rhee YS., Gong G., Cho YM, et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2005. 63:588–93.
37.Curtin JA., Fridlyand J., Kageshita T., Patel HN., Busam KJ., Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005. 353:2135–47.

36.Mitsutake N., Miyagishi M., Mitsutake S., Akeno N., Mesa C Jr., Knauf JA, et al. BRAF mediates RET/PTC-induced mitogen-activated protein kinase activation in thyroid cells: functional support for requirement of the RET/PTC-RAS-BRAF pathway in papillary thyroid carcinogenesis. Endocrinology. 2006. 147:1014–9.

38.Frasca F., Nucera C., Pellegriti G., Gangemi P., Attard M., Stella M, et al. BRAF (V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008. 15:191–205.
39.Guan H., Ji M., Bao R., Yu H., Wang Y., Hou P, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009. 94:1612–7.

40.Suarez HG. Molecular basis of epithelial thyroid tumorigenesis. C R Acad Sci III. 2000. 323:519–28.

41.Takahashi M., Ritz J., Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985. 42:581–8.

42.Fusco A., Grieco M., Santoro M., Berlingieri MT., Pilotti S., Pierotti MA, et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature. 1987. 328:170–2.

43.Grieco M., Santoro M., Berlingieri MT., Melillo RM., Donghi R., Bongarzone I, et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990. 60:557–63.

44.Santoro M., Dathan NA., Berlingieri MT., Bongarzone I., Paulin C., Grieco M, et al. Molecular characterization of RET/PTC3; a novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994. 9:509–16.
45.Miyagi E., Braga-Basaria M., Hardy E., Vasko V., Burman KD., Jhiang S, et al. Chronic expression of RET/PTC 3 enhances basal and insulin-stimulated PI3 kinase/AKT signaling and increases IRS-2 expression in FRTL-5 thyroid cells. Mol Carcinog. 2004. 41:98–107.

46.Ouyang B., Knauf JA., Smith EP., Zhang L., Ramsey T., Yusuff N, et al. Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clin Cancer Res. 2006. 12:1785–93.
48.Nikiforov YE. Radiation-induced thyroid cancer: what we have learned from chernobyl. Endocr Pathol. 2006. 17:307–17.

49.Thomas GA., Bunnell H., Cook HA., Williams ED., Nerovnya A., Cherstvoy ED, et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab. 1999. 84:4232–8.

50.Ishizaka Y., Kobayashi S., Ushijima T., Hirohashi S., Sugimura T., Nagao M. Detection of retTPC/PTC transcripts in thyroid adenomas and adenomatous goiter by an RT-PCR method. Oncogene. 1991. 6:1667–72.
51.Nikiforova MN., Caudill CM., Biddinger P., Nikiforov YE. Prevalence of RET/PTC rearrangements in Hashimoto's thyroiditis and papillary thyroid carcinomas. Int J Surg Pathol. 2002. 10:15–22.

52.Wirtschafter A., Schmidt R., Rosen D., Kundu N., Santoro M., Fusco A, et al. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis. Laryngoscope. 1997. 107:95–100.
53.Sheils OM., O'Eary JJ., Uhlmann V., Lattich K., Sweeney EC. Ret/PTC-1 Activation in hashimoto thyroiditis. Int J Surg Pathol. 2000. 8:185–9.

54.Elisei R., Romei C., Vorontsova T., Cosci B., Veremeychik V., Kuchinskaya E, et al. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endo-crinol Metab. 2001. 86:3211–6.

55.Fenton CL., Lukes Y., Nicholson D., Dinauer CA., Francis GL., Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000. 85:1170–5.
56.Adeniran AJ., Zhu Z., Gandhi M., Steward DL., Fidler JP., Giordano TJ, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006. 30:216–22.

57.Zhu Z., Ciampi R., Nikiforova MN., Gandhi M., Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006. 91:3603–10.
58.Chung KW., Chang MC., Noh DY., Oh SK., Choe KJ., Youn YK. RET oncogene expression of papillary thyroid carcinoma in Korea. Surg Today. 2004. 34:485–92.

59.Chung JH., Hahm JR., Min YK., Lee MS., Lee MK., Kim KW, et al. Detection of RET/PTC oncogene rearrangements in Korean papillary thyroid carcinomas. Thyroid. 1999. 9:1237–43.

60.Knauf JA., Kuroda H., Basu S., Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003. 22:4406–12.

61.Harvey JJ. An unidentified virus which causes the rapid production of tumours in mice. Nature. 1964. 204:1104–5.

62.Kirsten WH., Mayer LA. Morphologic responses to a murine erythroblastosis virus. J Natl Cancer Inst. 1967. 39:311–35.
63.Garcia-Rostan G., Zhao H., Camp RL., Pollan M., Herrero A., Pardo J, et al. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003. 21:3226–35.

64.Nikiforov YE. Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr Pathol. 2004. 15:319–27.

65.Lemoine NR., Mayall ES., Wyllie FS., Farr CJ., Hughes D., Padua RA, et al. Activated ras oncogenes in human thyroid cancers. Cancer Res. 1988. 48:4459–63.
66.Namba H., Rubin SA., Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990. 4:1474–9.

67.Vitagliano D., Portella G., Troncone G., Francione A., Rossi C., Bruno A, et al. Thyroid targeting of the N-ras(Gln61Lys) oncogene in transgenic mice results in follicular tumors that progress to poorly differentiated carcinomas. Oncogene. 2006. 25:5467–74.

68.Hihi AK., Michalik L., Wahli W. PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci. 2002. 59:790–8.
69.Kroll TG., Sarraf P., Pecciarini L., Chen CJ., Mueller E., Spiegelman BM, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science. 2000. 289:1357–60.
70.Marques AR., Espadinha C., Frias MJ., Roque L., Catarino AL., Sobrinho LG, et al. Underexpression of peroxisome proliferator-activated receptor (PPAR)gamma in PAX8/PPARgamma-negative thyroid tumours. Br J Cancer. 2004. 91:732–8.
71.French CA., Alexander EK., Cibas ES., Nose V., Laguette J., Faquin W, et al. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am J Pathol. 2003. 162:1053–60.

72.Nikiforova MN., Lynch RA., Biddinger PW., Alexander EK., Dorn GW 2nd., Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003. 88:2318–26.
73.Kim KY., Ahn JH., Cheon HG. Apoptotic action of peroxisome proliferator-activated receptor-gamma activation in human non small-cell lung cancer is mediated via proline oxidase-induced reactive oxygen species formation. Mol Pharmacol. 2007. 72:674–85.
74.Govindarajan R., Ratnasinghe L., Simmons DL., Siegel ER., Midathada MV., Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007. 25:1476–81.

75.Martelli ML., Iuliano R., Le Pera I., Sama I., Monaco C., Cammarota S, et al. Inhibitory effects of peroxisome poliferator-activated receptor gamma on thyroid carcinoma cell growth. J Clin Endocrinol Metab. 2002. 87:4728–35.
76.Giordano TJ., Au AY., Kuick R., Thomas DG., Rhodes DR., Wilhelm KG Jr, et al. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARG translocation. Clin Cancer Res. 2006. 12:1983–93.

77.Garcia-Rostan G., Costa AM., Pereira-Castro I., Salvatore G., Hernandez R., Hermsem MJ, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005. 65:10199–207.

78.Singh B., Reddy PG., Goberdhan A., Walsh C., Dao S., Ngai I, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002. 16:984–93.

79.Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005. 24:273–85.

80.Kada F., Saji M., Ringel MD. Akt: a potential target for thyroid cancer therapy. Curr Drug Targets Immune Endocr Metabol Disord. 2004. 4:181–5.

81.Ollikainen M., Gylling A., Puputti M., Nupponen NN., Abdel-Rahman WM., Butzow R, et al. Patterns of PIK3CA alterations in familial colorectal and endometrial carcinoma. Int J Cancer. 2007. 121:915–20.
82.Davies MA., Stemke-Hale K., Tellez C., Calderone TL., Deng W., Prieto VG, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008. 99:1265–8.

83.Liu Z., Hou P., Ji M., Guan H., Studeman K., Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008. 93:3106–16.

84.Carpten JD., Faber AL., Horn C., Donoho GP., Briggs SL., Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007. 448:439–44.
85.Liaw D., Marsh DJ., Li J., Dahia PL., Wang SI., Zheng Z, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997. 16:64–7.

86.Alvarez-Nunez F., Bussaglia E., Mauricio D., Ybarra J., Vilar M., Lerma E, et al. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid. 2006. 16:17–23.

87.Hou P., Liu D., Shan Y., Hu S., Studeman K., Condouris S, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007. 13:1161–70.

88.Halachmi N., Halachmi S., Evron E., Cairns P., Okami K., Saji M, et al. Somatic mutations of the PTEN tumor suppressor gene in sporadic follicular thyroid tumors. Genes Chromosomes Cancer. 1998. 23:239–43.
89.Wu G., Mambo E., Guo Z., Hu S., Huang X., Gollin SM, et al. Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab. 2005. 90:4688–93.
90.Ringel MD., Hayre N., Saito J., Saunier B., Schuppert F., Burch H, et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001. 61:6105–11.
91.Vasko V., Saji M., Hardy E., Kruhlak M., Larin A., Savchenko V, et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004. 41:161–70.

92.Yeager N., Klein-Szanto A., Kimura S., Di Cristofano A. Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res. 2007. 67:959–66.

93.Wang Y., Hou P., Yu H., Wang W., Ji M., Zhao S, et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/akt pathway in thyroid tumors. J Clin Endocrinol Metab. 2007. 92:2387–90.

94.Hollstein M., Sidransky D., Vogelstein B., Harris CC. p53 mutations in human cancers. Science. 1991. 253:49–53.

96.Freedman DA., Wu L., Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999. 55:96–107.

98.Tao W., Levine AJ. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci U S A. 1999. 96:6937–41.

99.Vogelstein B., Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004. 10:789–99. 100) Donghi R, Longoni A, Pilotti S, Michieli P, Della Porta G, Pierotti MA. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest 1993;91: 1753-60.

101.Boltze C., Roessner A., Landt O., Szibor R., Peters B., Schneider-Stock R. Homozygous proline at codon 72 of p53 as a potential risk factor favoring the development of undifferentiated thyroid carcinoma. Int J Oncol. 2002. 21:1151–4.

102.Saltman B., Singh B., Hedvat CV., Wreesmann VB., Ghossein R. Patterns of expression of cell cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery. 2006. 140:899–905. discussion 05-6.

103.Onel K., Cordon-Cardo C. MDM2 and prognosis. Mol Cancer Res. 2004. 2:1–8.
104.La Perle KM., Jhiang SM., Capen CC. Loss of p53 promotes anaplasia and local invasion in ret/PTC1-induced thyroid carcinomas. Am J Pathol. 2000. 157:671–7.

105.Mulholland DJ., Dedhar S., Coetzee GA., Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005. 26:898–915.
106.Van Aken E., De Wever O., Correia da Rocha AS., Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch. 2001. 439:725–51.

107.Garcia-Rostan G., Camp RL., Herrero A., Carcangiu ML., Rimm DL., Tallini G. Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol. 2001. 158:987–96.
108.Garcia-Rostan G., Tallini G., Herrero A., D'Aquila TG., Carcangiu ML., Rimm DL. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999. 59:1811–5.
109.Kurihara T., Ikeda S., Ishizaki Y., Fujimori M., Tokumoto N., Hirata Y, et al. Immunohistochemical and sequencing analyses of the Wnt signaling components in Japanese anaplastic thyroid cancers. Thyroid. 2004. 14:1020–9.

110.Mazzanti C., Zeiger MA., Costouros NG., Umbricht C., Westra WH., Smith D, et al. Using gene expression profiling to differentiate benign versus malignant thyroid tumors. Cancer Res. 2004. 64:2898–903.

111.Weber F., Shen L., Aldred MA., Morrison CD., Frilling A., Saji M, et al. Genetic classification of benign and malignant thyroid follicular neoplasia based on a three-gene combination. J Clin Endocrinol Metab. 2005. 90:2512–21.

112.Foukakis T., Gusnanto A., Au AY., Hoog A., Lui WO., Larsson C, et al. A PCR-based expression signature of malignancy in follicular thyroid tumors. Endocr Relat Cancer. 2007. 14:381–91.

113.Fan C., Oh DS., Wessels L., Weigelt B., Nuyten DS., Nobel AB, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006. 355:560–9.

114.Wang Y., Klijn JG., Zhang Y., Sieuwerts AM., Look MP., Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005. 365:671–9.

Fig. 1
Thyroid carcinoma-related signal pathways. Proto-onco-genes and oncogenes are shaded in the diagram. Rectangular figures represent membrane proteins, and round figures cytoplasmic proteins.
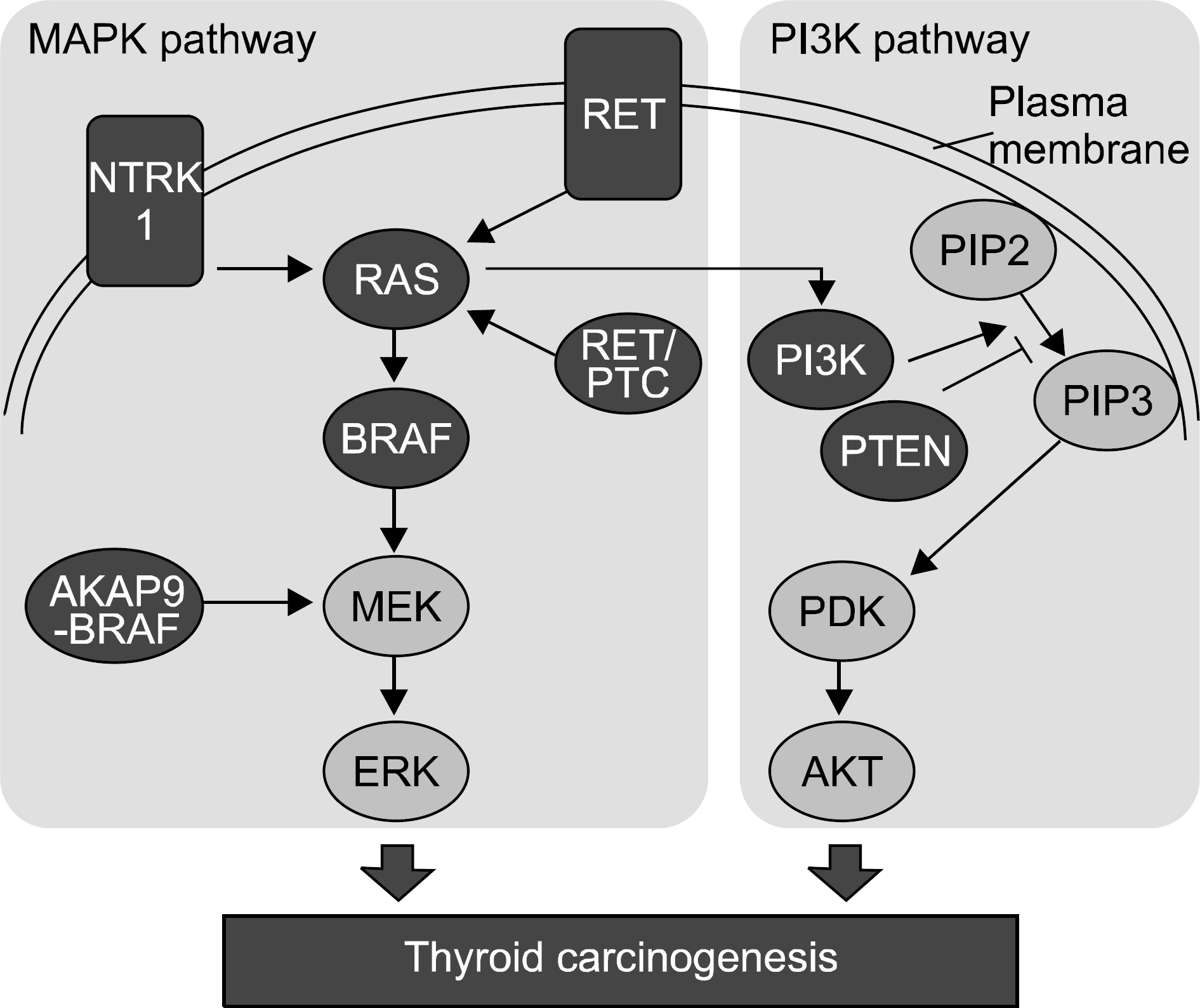
Table 1.
Genetic rearrangements found in papillary thyroid carcinomas




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download