Abstract
Purpose
Chronic nonbacterial prostatitis and chronic pelvic pain syndrome account for 90-95% of all prostatitis. Little is known about its pathophysiology, thus, various treatments are used. Ecklonia cava, a seaweed, is a member of the brown algae family; many recent reports have demonstrated that its extract containing phlorotannin has anti-oxidative and anti-inflammatory properties. Using the hormone-induced prostatitis rat model, we investigated the anti-inflammatory effects of E. cava extracts via its anti-oxidative process on chronic nonbacterial prostatitis.
Materials and Methods
Forty, 10-week-old male white Wistar rats were utilized, and divided equally into the following five groups: 1) control, 2) E. cava-fed, 3) hormone-induced prostatitis (HIP), 4) E. cava-treated HIP, and 5) nonsteroidal anti-inflammatory drug (NSAID)-treated HIP.
Results
The results showed statistically-significant improvement in the tissue response to the hormone-induced inflammation among the E. cava-treated and NSAID-treated groups (p<0.05). Lower malonedialdehyde levels were observed in the group with E. cava-treated HIP than with HIP alone, which was statistically significant. We believe that this supports the anti-oxidative properties of E. cava.
Go to : 
REFERENCES
1. Nickel JC, Nyberg LM, Hennenfent M. Research guidelines for chronic prostatitis: consensus report from the first National Institutes of Health International Prostatitis Collaborative Network. Urology. 1999; 54:229–33.

2. Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001; 165:842–5.

3. Bahk JY, Hyun JS, Lee JY, Kim J, Cho YH, Lee JH, et al. Concentration of ofloxacin in canine prostate tissue and prostate fluid after intraprostatic injection of biodegradable sustained-releasing microspheres containing ofloxacin. J Urol. 2000; 163:1560–4.

5. Wijesinghe WA, Jeon YJ. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: a review. Int J Food Sci Nutr. 2012; 63:225–35.
6. Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988; 12:271–86.

7. Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988; 140:1049–53.

8. Kim DS, Lee EJ, Cho KS, Yoon SJ, Lee YH, Hong SJ. Preventive effects of oligomerized polyphenol on estradiol-induced prostatitis in rats. Yonsei Med J. 2009; 50:391–8.

9. Seo SI, Lee SJ, Kim JC, Choi YJ, SW SW, Hwang TK, et al. Effects of androgen deprivation on chronic bacterial prostatitis in a rat model. Int J Urol. 2003; 10:485–91.

10. Gerard-Monnier D, Erdelmeier I, Regnard K, Moze-Henry N, Yadan JC, Chaudiere J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998; 11:1176–83.
11. Kang HS, Chung HY, Jung JH, Son BW, Choi JS. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem Pharm Bull (Tokyo). 2003; 51:1012–4.

12. Kang KA, Lee KH, Chae S, Koh YS, Yoo BS, Kim JH, et al. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic Res. 2005; 39:883–92.
13. Wijesekara I, Yoon NY, Kim SK. Phlorotannins from Ecklonia cava (Phaeophyceae): biological activities and potential health benefits. Biofactors. 2010; 36:408–14.

14. Shull JD, Gorski J. Regulation of prolactin gene transcription in vivo: interactions between estrogen, pimozide, and alpha-er-gocryptine. Mol Pharmacol. 1990; 37:215–21.
15. Tangbanluekal L, Robinette CL. Prolactin mediates estradiol-induced inflammation in the lateral prostate of Wistar rats. Endocrinology. 1993; 132:2407–16.

16. Wilson MJ, Woodson M, Wiehr C, Reddy A, Sinha AA. Matrix metalloproteinases in the pathogenesis of estradiol-induced nonbacterial prostatitis in the lateral prostate lobe of the Wistar rat. Exp Mol Pathol. 2004; 77:7–17.

17. Arakawa S, Matsui T, Gohji K, Okada H, Kamidono S. Prostatitis–the Japanese viewpoint. Int J Antimicrob Agents. 1999; 11:201–3.

18. Maher J, Yamamoto M. The rise of antioxidant signaling–the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010; 244:4–15.

19. Heo SJ, Park PJ, Park EJ, Kim SK, Jeon YJ. Antioxidant activity of enzymatic extracts from a brown seaweed Ecklonia cava by electron spin resonance spectrometry and comet assay. Eur Food Res Technol. 2005; 221:41–7.

20. Yasmeen T, Qureshi GS, Perveen S. Adverse effects of diclofenac sodium on renal parenchyma of adult albino rats. J Pak Med Assoc. 2007; 57:349–51.
21. Van Hecken A, Schwartz JI, Depre M, De Lepeleire I, Dallob A, Tanaka W, et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000; 40:1109–20.
Go to : 
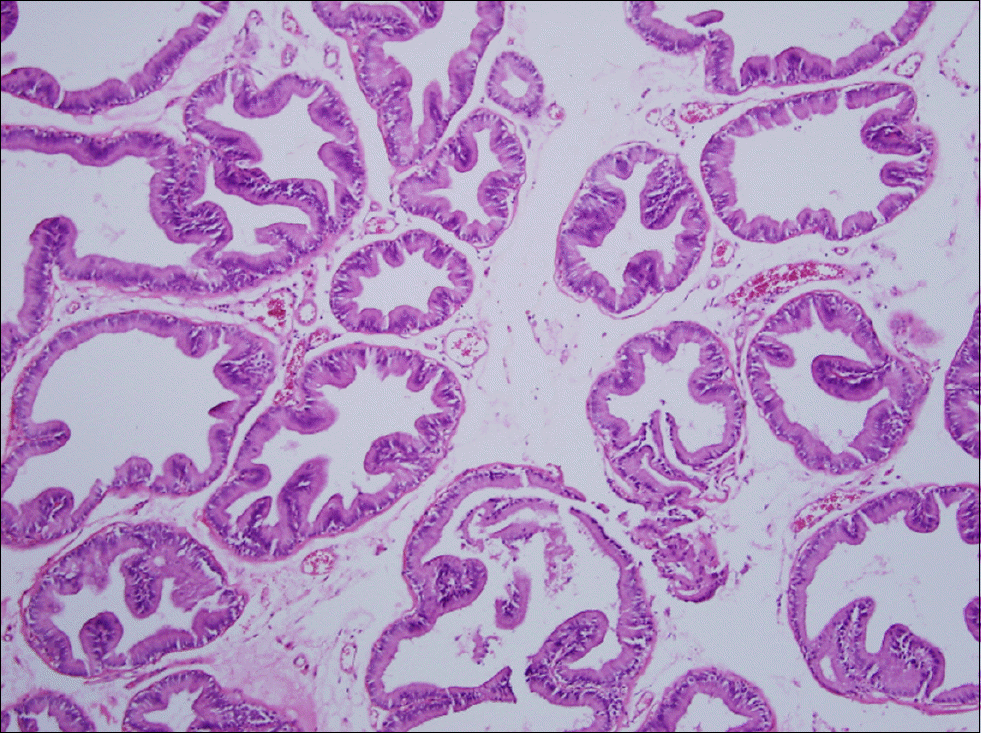 | Fig. 1.Prostate section of control group. There is no evidence of chronic inflammatory cell infiltration, acinar change and interstitial fibrosis (H&E stain, ×100). |
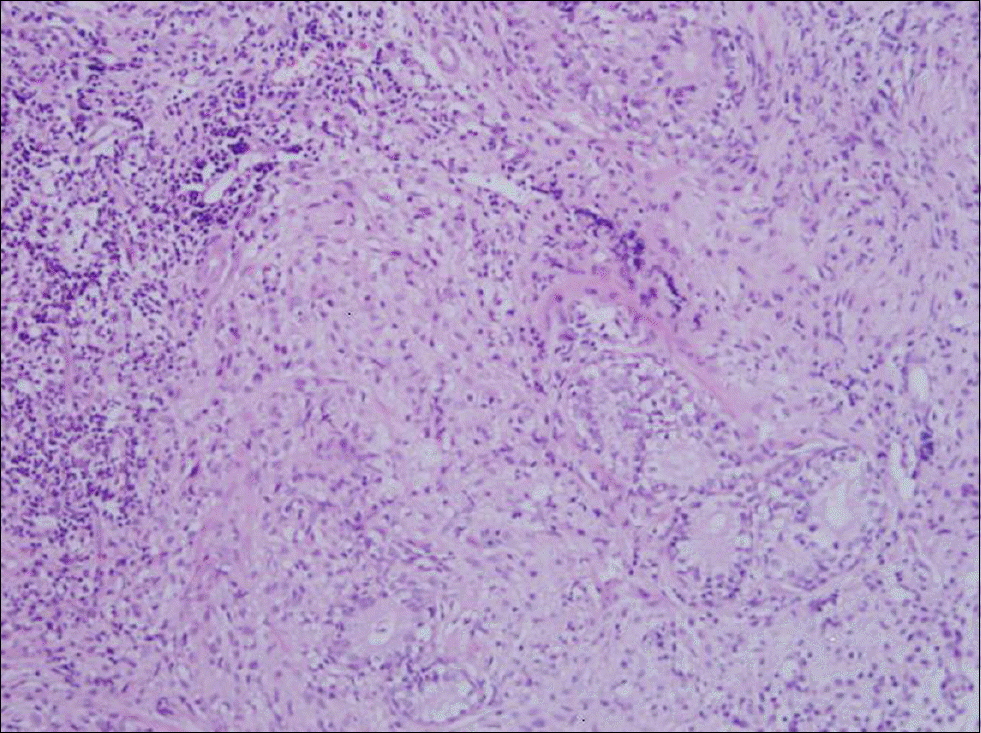 | Fig. 2.Prostate section of hormone-induced prostatitis group. Severe chronic inflammatory cell infiltration and interstitial fibrosis are seen. The acinar structures are severely atrophied (H&E stain, ×100). |
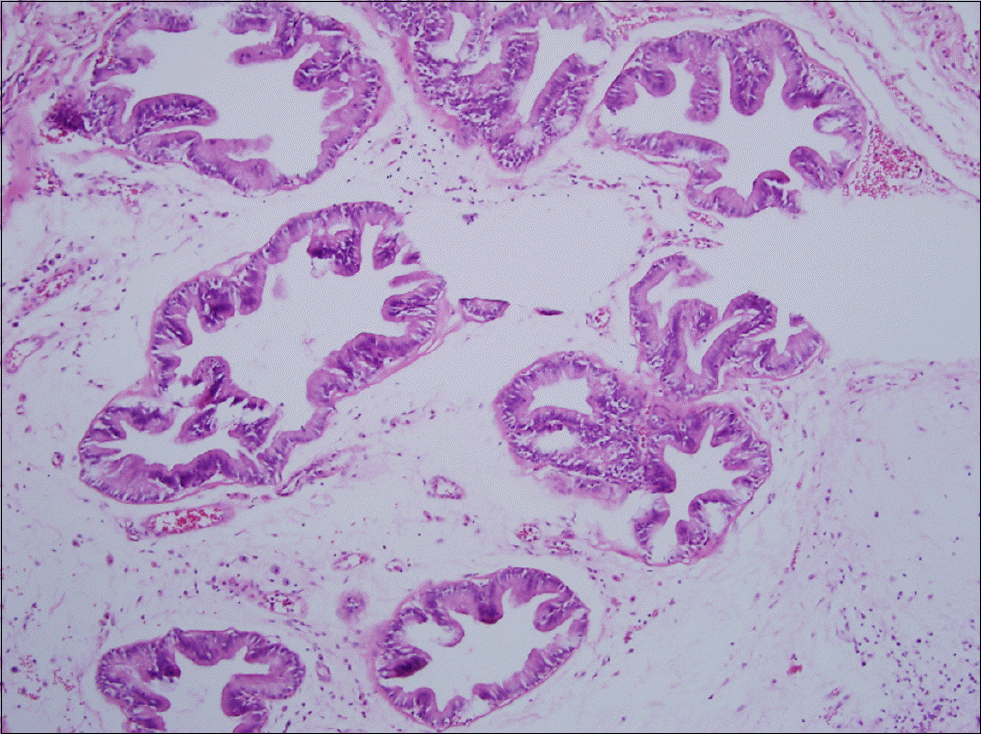 | Fig. 3.Prostate section of Ecklonia cava-treated group. Mild infiltration of chronic inflammatory cells, mildly atrophied acini, and mild interstitial fibrosis are seen (H&E stain, ×100). |
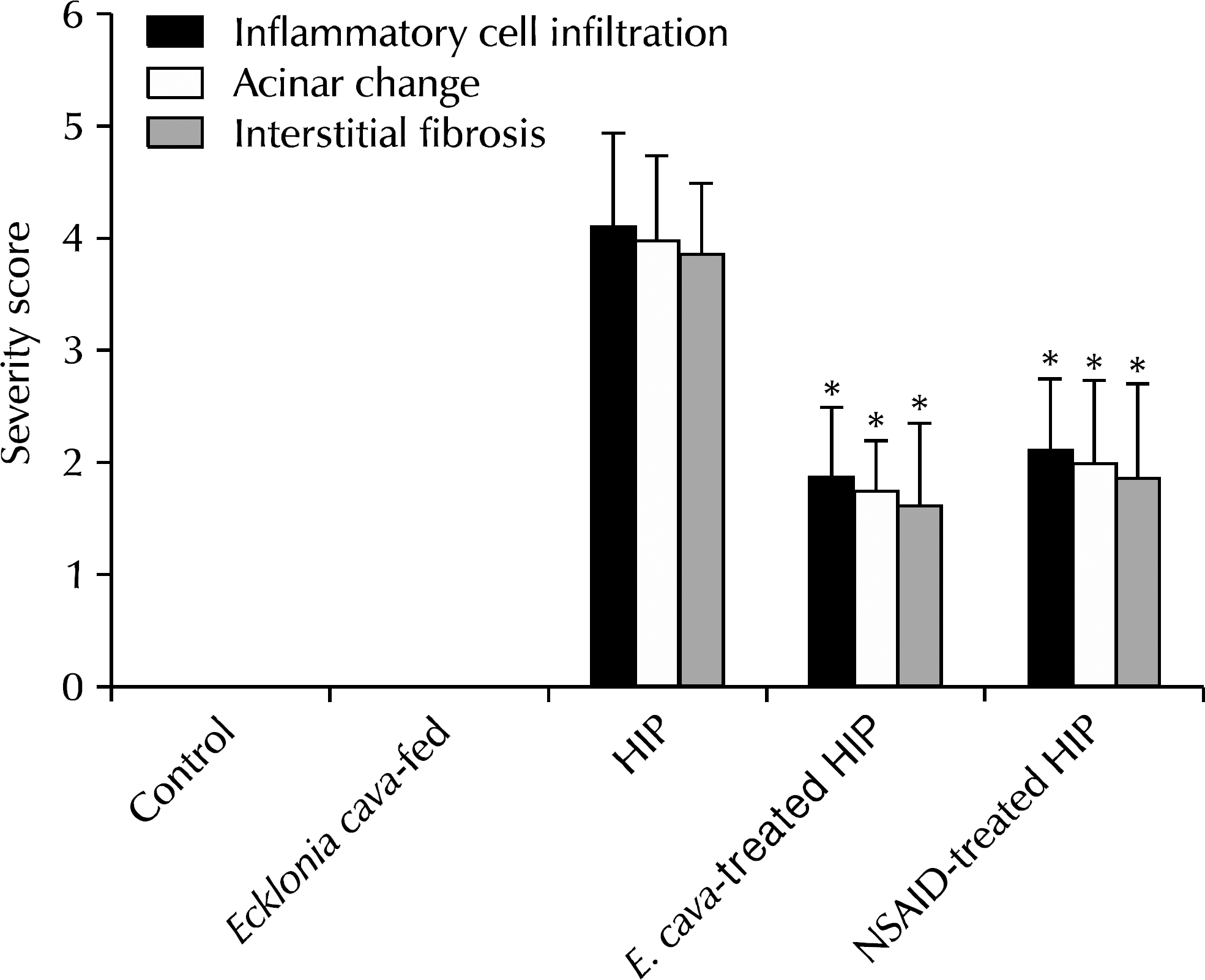 | Fig. 4.Severity scores of chronic inflammatory cell infiltration, acinar changes and interstitial fibrosis in each group. ∗p<0.05 compared with hormone-induced prostatitis (HIP) group. NSAID: nonsteroidal antiinflammatory drug. |
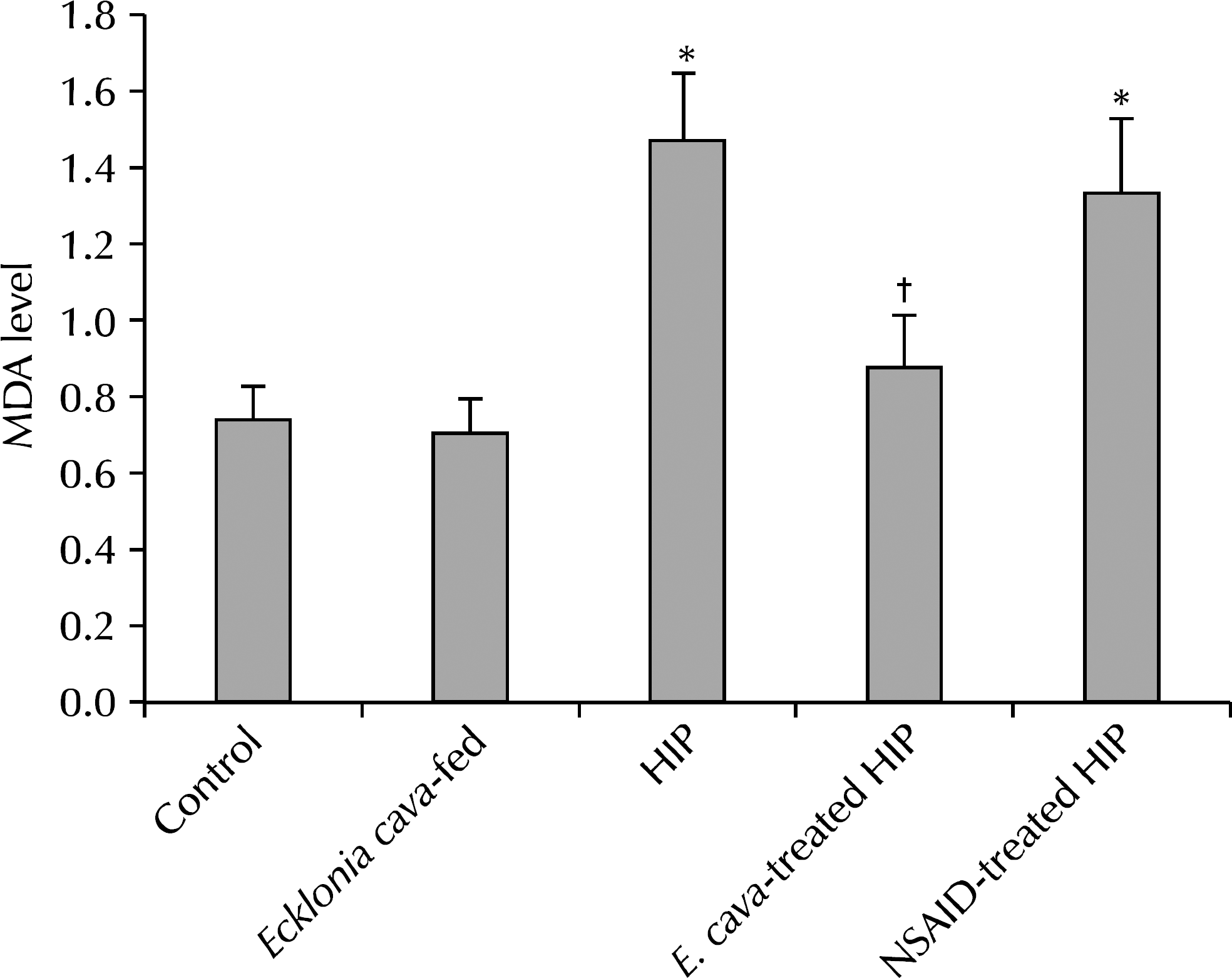 | Fig. 5.Mean malonedialdehyde (MDA) values measured in each group. ∗p<0.05 compared with control group; †p<0.05 compared with hormone-induced prostatitis (HIP) group. NSAID: nonsteroidal antiinflammatory drug. |
Table 1.
Severity scores of chronic inflammatory cell infiltrations, acinar changes and interstitial fibrosis of prostate tissue
Table 2.
Severity scores of chronic inflammatory cell infiltrations, acinar changes and interstitial fibrosis in each group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download