Abstract
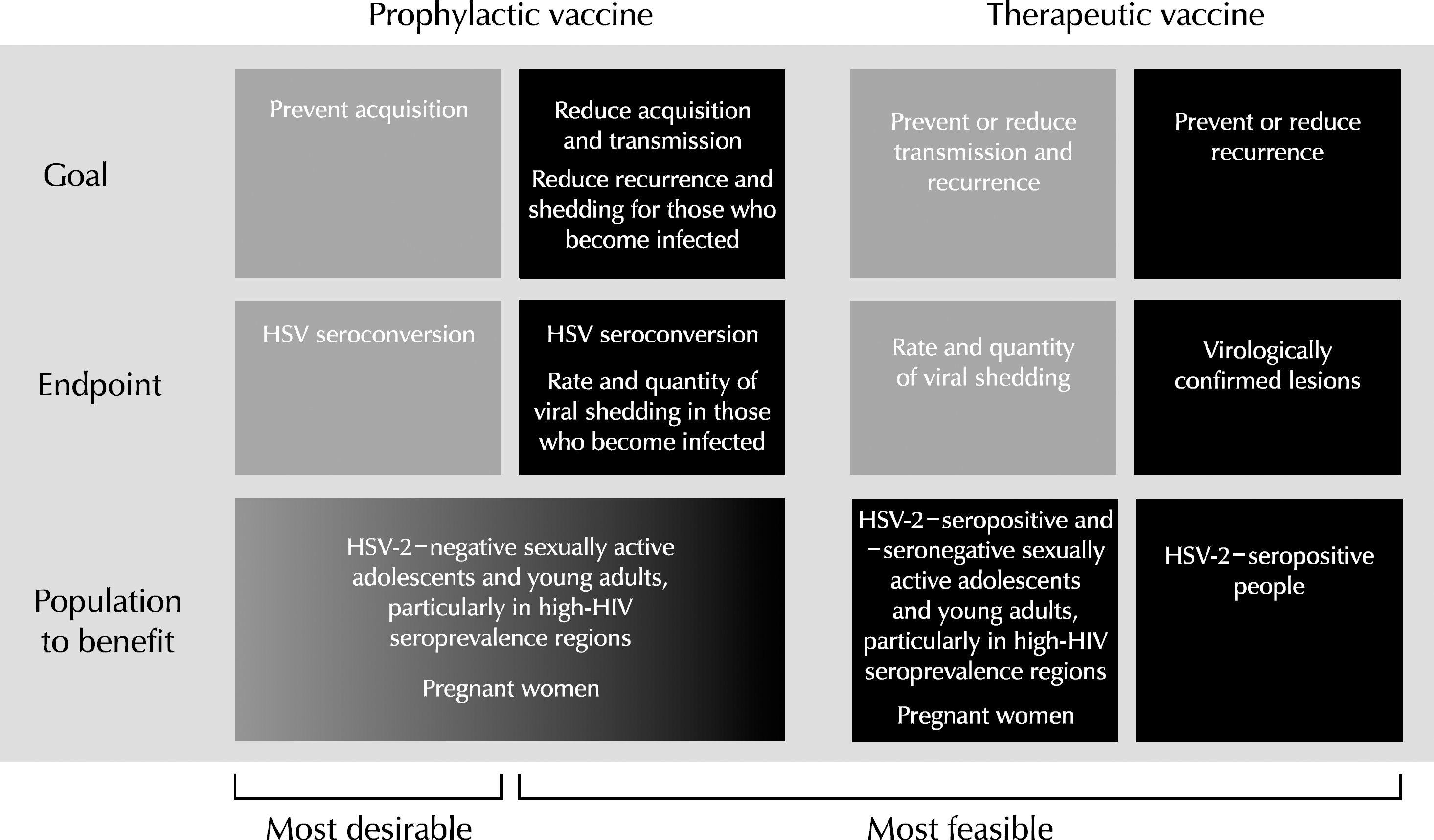
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2), also known as human herpes virus 1 and 2 (HHV-1 and HHV-2), are two members of the herpes virus family, herpes viridae, which infect humans. Both HSV-1 (which produces most cold sores) and HSV-2 (which produces most genital herpes) are ubiquitous and contagious. They can be spread when an infected person is producing and shedding the virus. Herpes Simplex can be spread through contact with saliva, such as sharing drinks. HSV-2 is one of the most prevalent sexually transmitted infections worldwide. In addition to recurrent genital ulcers, HSV-2 causes neonatal herpes, and is associated with a 3-fold increased risk for HIV acquisition. Many HSV-2 vaccines have been studied in animal models, however, few have reached clinical trials, and those that have been tested in humans were not consistently effective. Here, I review HSV-2 pathogenesis, with a focus on novel understanding of mucosal immunobiology of HSV-2, and vaccine efforts to date, in an attempt to stimulate thinking about future directions for development of effective prophylactic and therapeutic HSV-2 vaccines.
REFERENCES
1. Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008; 86:805–12. A.

2. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and metaanalysis of longitudinal studies. AIDS. 2006; 20:73–83.

3. Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, et al. ANRS 1285 Study Group. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007; 356:790–9.

5. Koelle DM, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Microbiol Rev. 2003; 16:96–113.

6. Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Bada-khshan T, Kim SM, et al. Future of an "asymptomatic" T-cell epitope-based therapeutic herpes simplex vaccine. Future Virol. 2012; 7:371–8.

7. Corey L, Bodsworth N, Mindel A, Patel R, Schacker T, Stanberry L. An update on short-course episodic and prevention therapies for herpes genitalis. Herpes. 2007; 14(Suppl 1):5A–11A.
8. Field HJ, Darby G, Wildy P. Isolation and characterization of acyclovir-resistant mutants of herpes simplex virus. J Gen Virol. 1980; 49:115–24.

9. Semenova TB, Posevaia TA, Vanag AI, Barinskii IF. Properties of herpes simplex virus strains isolated from patients with recurrent cutaneous herpes. Vopr Virusol. 1985; 30:93–6.
10. Dundarov S, Andonov P, Bakalov B. Characterization of herpes simplex virus strains isolated from patients with various diseases. Arch Virol. 1980; 63:115–21.

11. Skinner GR, Davies JA, Dundarov S, Andonov P. Prevention of herpes genitalis by the 'Bulgarian' vaccine F.HSV-2V(PRK): preliminary clinical evidence. Croat Med J. 2000; 41:378–83.
12. Wachsman M, Kulka M, Smith CC, Aurelian L. A growth and latency compromised herpes simplex virus type 2 mutant (ICP10DeltaPK) has prophylactic and therapeutic protective.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download