Abstract
Purpose
Vaginal epithelial cells have always been exposed to various pathogens. However, this has not always caused clinical infection. In addition to a previously reported protection effect of the vagina, currently, the innate immune response is thought to be important as one of the causes explaining the phenomenon. Therefore, we investigated the innate immunity of the vagina and related mechanisms in infected vaginal epithelial cells focusing on the antimicrobial peptide human β-defensin-3 (HBD-3).
Materials and Methods
We investigated the signaling molecules, Toll-like receptors (TLRs), through which mammals sense infection in vaginal epithelial cells, with activation with lipopolysaccharide (LPS), Staphylococcus aureus peptidoglycan (PGN), or zymosan. Reverse transcriptase-polymerase chain reaction analysis of HBD-3 messenger RNA expression in vaginal epithelial cells after treatment with three pathogens was performed for investigation of pathogen-associated molecular patterns. Then, we also studied the following mechanism of innate immunity of the vagina focusing on HBD-3 in vaginal epithelial cells infected with gram-positive bacteria, gram-negative bacteria, or fungus.
Results
Vaginal epithelial cells (VK2/E6E7 cells) constitutively expressed TLR2 and TLR4 and produced antimicrobial peptide HBD-3 upon activation with LPS, PGN, or zymosan. VK2/E6/E7 cells exposed to LPS, PGN, or zymosan showed increased p38 mitogen activated protein kinase (MAPK) activity. In addition, LPS-, PGN-, and zymosan-induced HBD-3 expression was attenuated by SB203580, a p38 MAPK inhibitor, emphasizing the importance of p38 MAPK in induction of HBD-3.
Go to : 
REFERENCES
1. Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990; 12:856–72.

2. Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, et al. Microbial compounds induce the expression of proinflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005; 7:1117–27.
3. Hertzman M. Galantamine in the treatment of adult autism: a report of three clinical cases. Int J Psychiatry Med. 2003; 33:395–8.

4. Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011; 8:388–403.

5. Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004; 5:1061–8.
6. Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismüller KH, Godowski PJ, et al. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003; 171:6820–6.
7. Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999; 274:7611–4.
8. Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004; 6:1382–7.

9. Gayer G, Mini S, Olchovsky D, Leibovitch I, Apter S, Weitzen R, et al. Pulmonary embolism-the initial manifestation of renal cell carcinoma in a young woman. Emerg Radiol. 2003; 10:43–5.

10. Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985; 76:1427–35.

12. Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997; 387:861.

13. Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000; 275:29731–6.
14. Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001; 276:5707–13.
15. Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, et al. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000; 22:714–21.
16. Ishimoto H, Mukae H, Date Y, Shimbara T, Mondal MS, Ashitani J, et al. Identification of hBD-3 in respiratory tract and serum: the increase in pneumonia. Eur Respir J. 2006; 27:253–60.

17. Maisetta G, Batoni G, Esin S, Florio W, Bottai D, Favilli F, et al. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob Agents Chemother. 2006; 50:806–9.
19. Strus M, Malinowska M, Heczko PB. In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. J Reprod Med. 2002; 47:41–6.
20. Nomanbhoy F, Steele C, Yano J, Fidel PL Jr. Vaginal and oral epithelial cell anti-Candida activity. Infect Immun. 2002; 70:7081–8.
21. Steele C, Ozenci H, Luo W, Scott M, Fidel PL Jr. Growth inhibition of Candida albicans by vaginal cells from naïve mice. Med Mycol. 1999; 37:251–9.

22. Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001; 2:675–80.

23. Han JH, Kim MS, Lee MY, Kim TH, Lee MK, Kim HR, et al. Modulation of human beta-defensin-2 expression by 17beta-estradiol and progesterone in vaginal epithelial cells. Cytokine. 2010; 49:209–14.
24. Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pità O, Leung DY, et al. IL-4 and IL-13 negatively regulate TNF-alpha-and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007; 179:984–92.
25. Menzies BE, Kenoyer A. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin 3 in skin keratinocytes. Infect Immun. 2006; 74:6847–54.
26. Steubesand N, Kiehne K, Brunke G, Pahl R, Reiss K, Herzig KH, et al. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol. 2009; 10:36.

27. Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003; 23:2871–82.
Go to : 
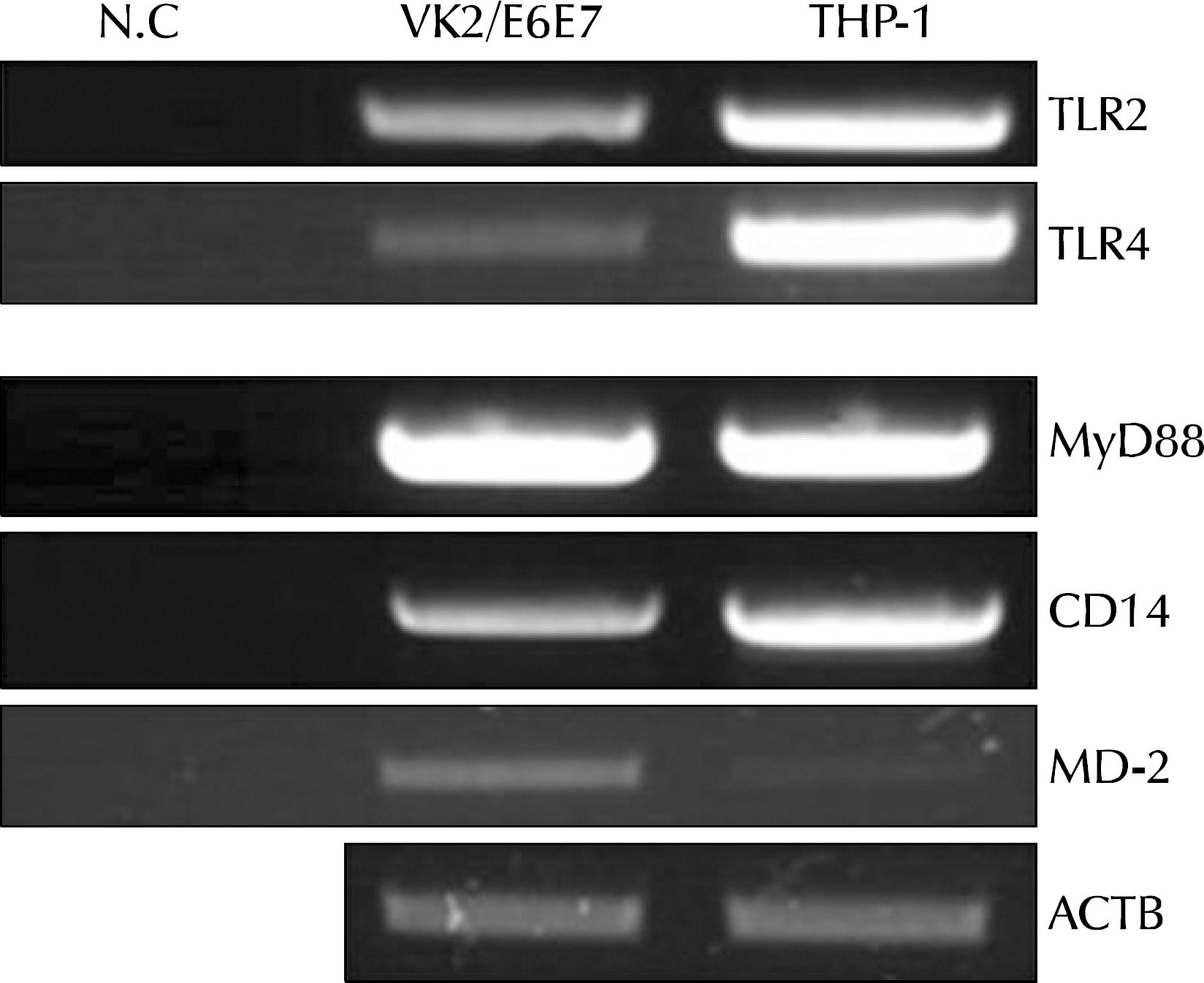 | Fig. 1.Expression of Toll-like receptors (TLRs) and accessory molecules in cultured human vaginal epithelial cells. VK2/E6E7, a normal human vaginal epithelial cell line, was screened for mRNA expression of all known human TLRs and the accessory molecules myeloid differentiation protein 2 (MD-2), CD14, and myeloid differentiation primary response gene 88 (MyD88) by reverse transcriptase-polymerase chain reaction. The expression of TLR2 and TLR4 mRNA was detected in VK2/E6E7 cells. The expression of accessory molecules such as MyD88, CD14, and MD-2 was also detected. N.C: normal control, ACTB: β-actin. |
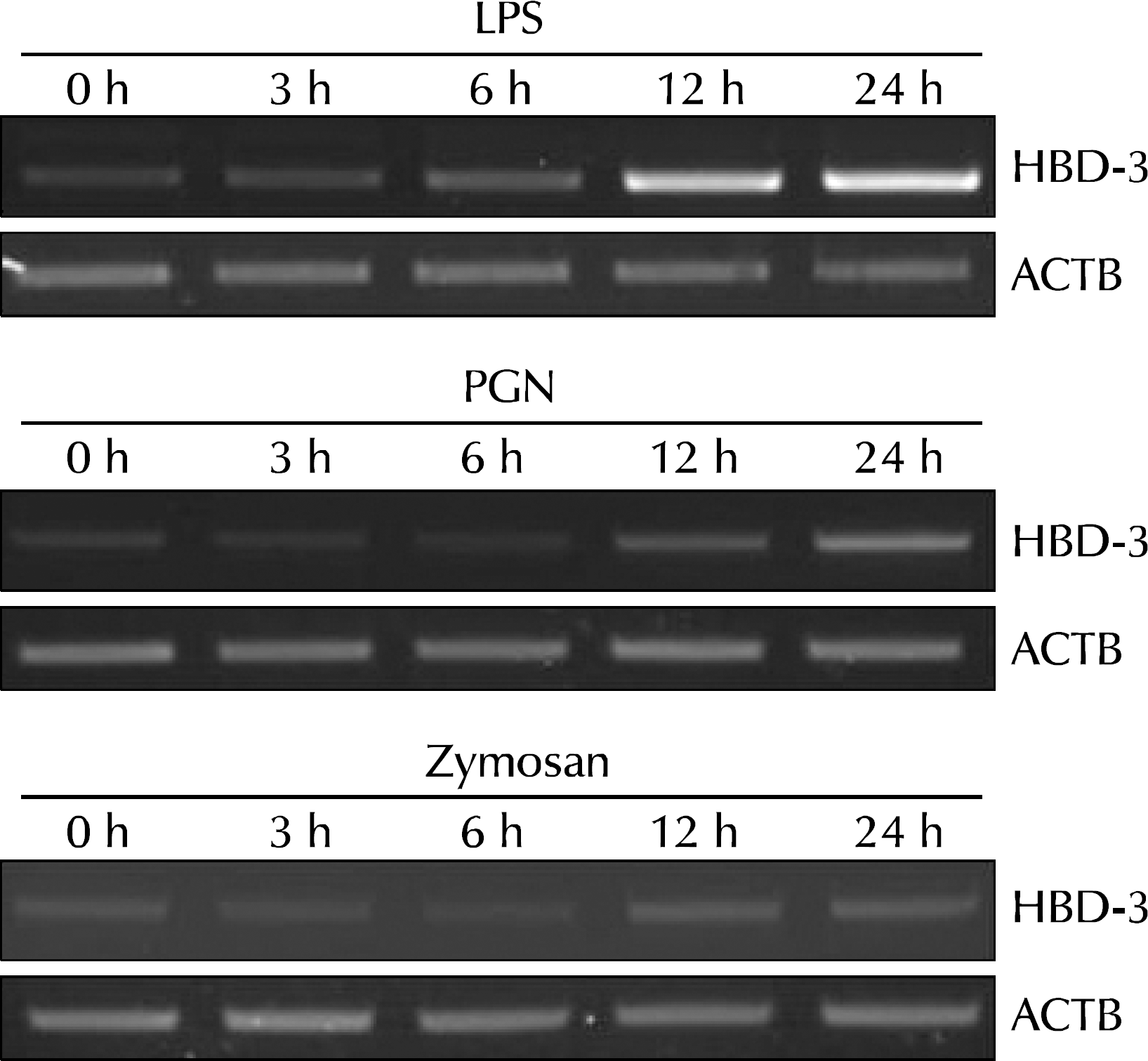 | Fig. 2.Pathogen-associated molecular patterns, representing gram-negative and gram-positive bacteria as well as fungal pathogens, induced human β-defensin-3 (HBD-3) in vaginal epithelial cells. Semiquantitative reverse transcriptase-polymerase chain reaction analysis of HBD-3 mRNA expression in immortalized vaginal epithelial cells after treatment with lipopolysaccharide (LPS), Staphylococcus aureus peptidoglycan (PGN), or zymosan was performed. LPS, PGN, and zymosan had an extremely strong stimulatory effect on HBD-3 mRNA expression in VK2/E6E7 cells at 3, 6, 12, and 24 hours after stimulation. ACTB: β-actin. |
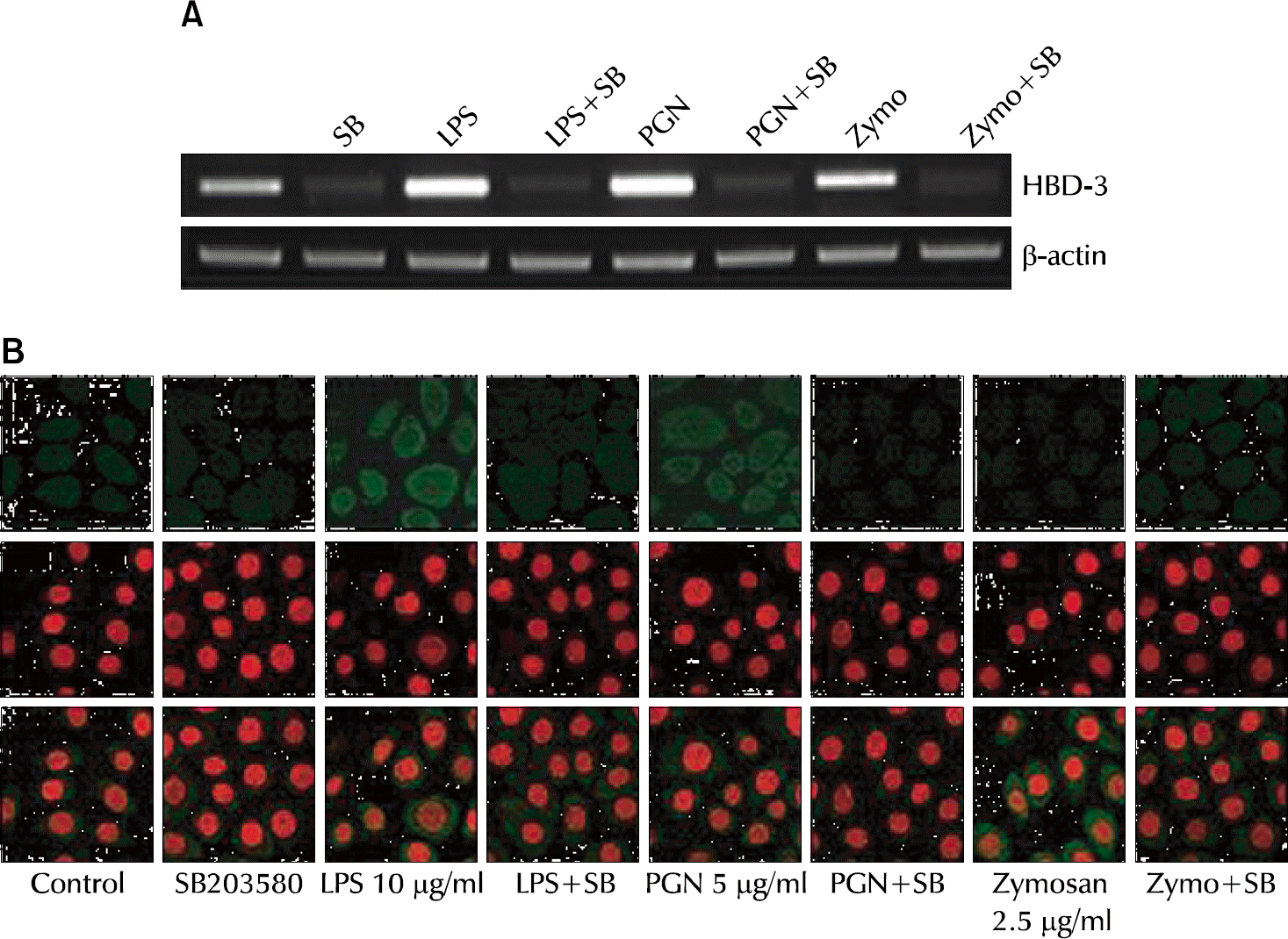 | Fig. 3.The specific inhibitor of p38 MAPK (SB203580) attenuates lipopolysaccharide (LPS)-, Staphylococcus aureus peptidoglycan (PGN)-, and zymosan-induced human beta-defensin-3 (HBD-3) expression in VK2/E6E7 cells in the mRNA level (A) and the protein level (B). |
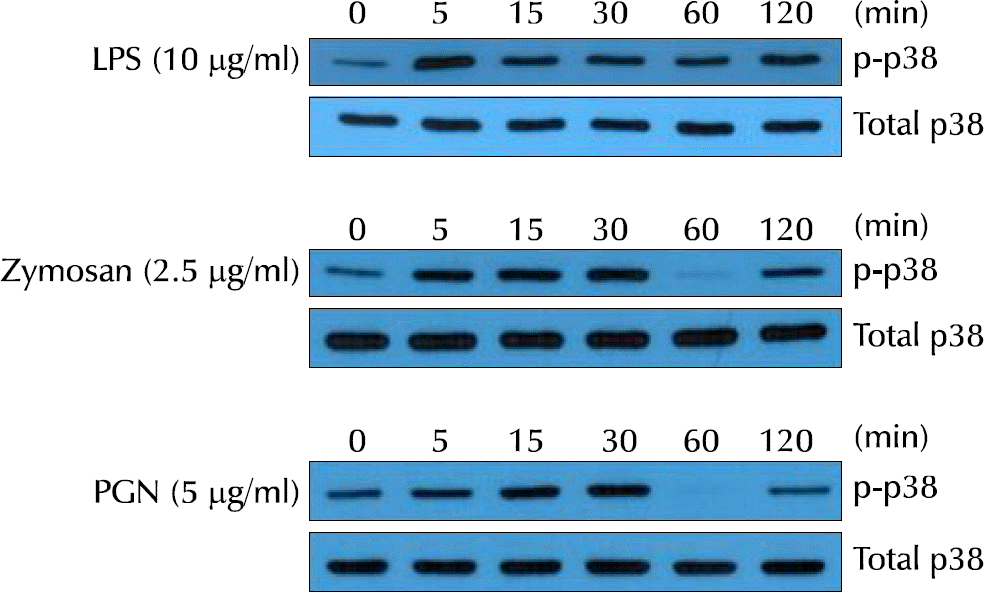 | Fig. 4.Lipopolysaccharide (LPS), Staphylococcus aureus peptidoglycan (PGN), and zymosan induced p38 mitogen-activated protein kinase (MAPK) phosphorylation in VK2/E6E7 cells. The activation of p38 MAPK by detecting the phosphor-p38 MAPK protein was observed. |
Table 1.
Primer sets used in polymerase chain reaction analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download