Abstract
Gonorrhea, caused by Neisseria gonorrhoeae is the second most prevalent bacterial sexually transmitted infection. The disease causes serious reproductive complications such as pelvic inflammatory disease, ectopic pregnancy, and infertility, and can facilitate human immunodeficiency virus transmission. Numerous antimicrobial agents have been used for the treatment of gonorrhea since sulfanilamides were introduced in 1936. Unfortunately, N. gonorrhoeae readily develops resistance to antimicrobial agents. Strains with decreased susceptibility to oral third generation cephalosporin (cefixime) are currently emerging. The US Centers for Disease Control and Prevention (CDC) no longer recommends cefixime at any dose as a first-line regimen for treatment of gonococcal infections, but recommends combination therapy with ceftriaxone 250 mg intramuscularly and either azithromycin 1 g orally as a single dose or doxycycline 100 mg orally twice daily for 7 days as the most reliably effective treatment for uncomplicated gonorrhea.
REFERENCES
1. Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999; 75:3–17.

2. Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2009; 7:821–34.
3. Centers for Disease Control and Prevention (CDC). Update to CDC's Sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012; 61:590–4.
4. Lee KW. Study on antimicrobial resistance of Neisseria gonorrhoeae isolated in Korea (6th year) [Internet]. Cheongwon: Korea Centers for Disease Control & Prevention;2011. [cited 2013 Mar 4]. Available from:. http://www.cdc.go.kr/CDC/info/CdcKrInfo0201.jsp?menuIds=HOME001-MNU0004-MNU0007-MNU0025&fid=28&q_type=title&q_value=%EC%9E%84%EA%B7%A0&cid=1805&pageNum.
5. Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, Serwadda D, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? Int J STD AIDS. 2002; 13:91–101.

6. Lim DH, Lee SJ, Shim BS, Kim CS, Kim ME, Cho YH. The new Korean guideline for sexually transmitted infections. Korean J Urogenit Tract Infect Inflamm. 2011; 6:96–113.
7. Benedek TG. History of the medical treatment of gonorrhea. Available from:. http://www.antimicrobe.org/h04c.files/history/Gonorrhea.asp.
8. Workowski KA, Berman SM, Douglas JM Jr. Emerging anti-microbial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann Intern Med. 2008; 148:606–13.

10. Centers for Disease Control (CDC). CDC recommended treatment schedules, 1974. MMWR Morb Mortal Wkly Rep. 1974; 23:341–7.
11. Centers for Disease Control (CDC). 1985 STD Treatment Guidelines. MMWR Morb Mortal Wkly Rep. 1985; 34(Suppl 4):75S–108S.
12. Sexually transmitted diseases treatment guidelines. MMWR Morb Mortal Wkly Rep. 1989; 38(Suppl 8):1–43.
13. Sexually transmitted diseases treatment guidelines. Centers for disease control and prevention. MMWR Morb Mortal Wkly Rep. 1993; 42:1–102.
14. Iverson CJ, Wang SA, Lee MV, Ohye RG, Trees DL, Knapp JS, et al. Fluoroquinolone resistance among Neisseria gonorrhoeae isolates in Hawaii, 1990-2000: role of foreign importation and increasing endemic spread. Sex Transm Dis. 2004; 31:702–8.
15. Centers for Disease Control and Prevention (CDC). Fluoroquinolone-resistance in Neisseria gonorrhoeae, Hawaii, 1999, and decreased susceptibility to azithromycin in N. gonorrhoeae, Missouri, 1999. MMWR Morb Mortal Wkly Rep. 2000; 49:833–7.
16. Centers for Disease Control and Prevention (CDC). Increases in fluoroquinolone-resistant Neisseria gonorrhoeae–Hawaii and California, 2001. MMWR Morb Mortal Wkly Rep. 2002; 51:1041–4.
17. Centers for Disease Control and Prevention (CDC). Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men–United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep. 2004; 53:335–8.
18. Centers for Disease Control and Prevention (CDC). Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007; 56:332–6.
19. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: are cephalosporins next? Curr Infect Dis Rep. 2011; 13:196–204.

20. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert Opin Phar-macother. 2009; 10:555–77.

21. Yokoi S, Deguchi T, Ozawa T, Yasuda M, Ito S, Kubota Y, et al. Threat to cefixime treatment for gonorrhea. Emerg Infect Dis. 2007; 13:1275–7.
22. Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill. 2010; 15:pii: 19721.

23. Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011; 17:148–9.
24. Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011; 55:3538–45.

25. Unemo M, Golparian D, Potočnik M, Jeverica S. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill. 2012; 17:pii: 20200.

26. Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill. 2011; 16:pii: 19792.

27. Centers for Disease Control and Prevention (CDC). Gonococcal isolate surveillance project, GISP Profiles 2011 Introduction. Available from:. http://www.cdc.gov/std/gisp2011/GISP2011ExplanationFull.pdf.
28. Tapsall JW. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 1998. The WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Commun Dis Intell. 2000; 24:1–4.
29. Ito M, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, et al. Remarkable increase in central Japan in 2001-2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrob Agents Chemother. 2004; 48:3185–7.
30. WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2004. Commun Dis Intell Q Rep. 2006; 30:129–32.
31. Lahra MM. WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2010. Commun Dis Intell Q Rep. 2012; 36:95–100.
32. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59:1–110.
33. Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis. 1995; 20(Suppl 1):S47–65.

34. Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis. 2007; 44(Suppl 3):S84–101.

35. Sathia L, Ellis B, Phillip S, Winston A, Smith A. Pharyngeal gonorrhoea-is dual therapy the way forward? Int J STD AIDS. 2007; 18:647–8.
36. Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill. 2011; 16:pii: 19833.

37. Unemo M, Golparian D, Stary A, Eigentler A. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill. 2011; 16:pii: 19998.

38. Yasuda M, Fukuda H, Yokoi S, Ishihara S, Kawada Y, Deguchi T. In vitro selection of fluoroquinolone-resistant Neisseria gonorrhoeae harboring alterations in DNA gyrase and topoisomerase IV. J Urol. 2000; 164:847–51.

39. Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, et al. Mosaic-like structure of penicillin-binding protein 2 Gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother. 2002; 46:3744–9.
40. Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother. 2005; 49:137–43.
41. Deguchi T, Yasuda M, Maeda S. Lack of nationwide surveillance of antimicrobial resistance of Neisseria gonorrhoeae in Japan. Ann Intern Med. 2008; 149:363–4.

Fig. 2.
Annual trend of cefixime minimum inhibitory concentrations of Neisseria gonorrhoeae isolated in Korea.
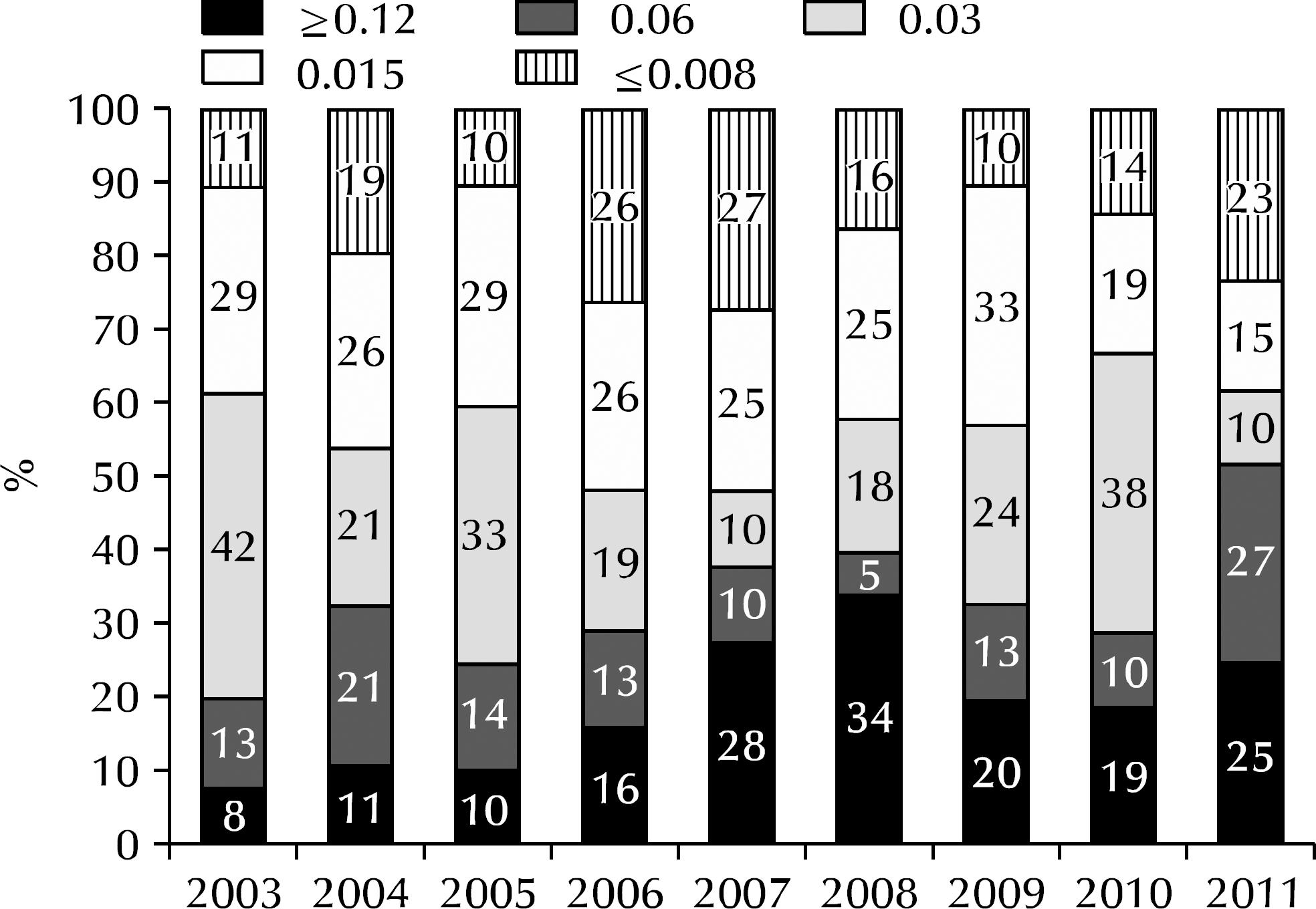
Fig. 3.
Annual trend of ceftriaxone minimum inhibitory concentrations of Neisseria gonorrhoeae isolated in Korea.
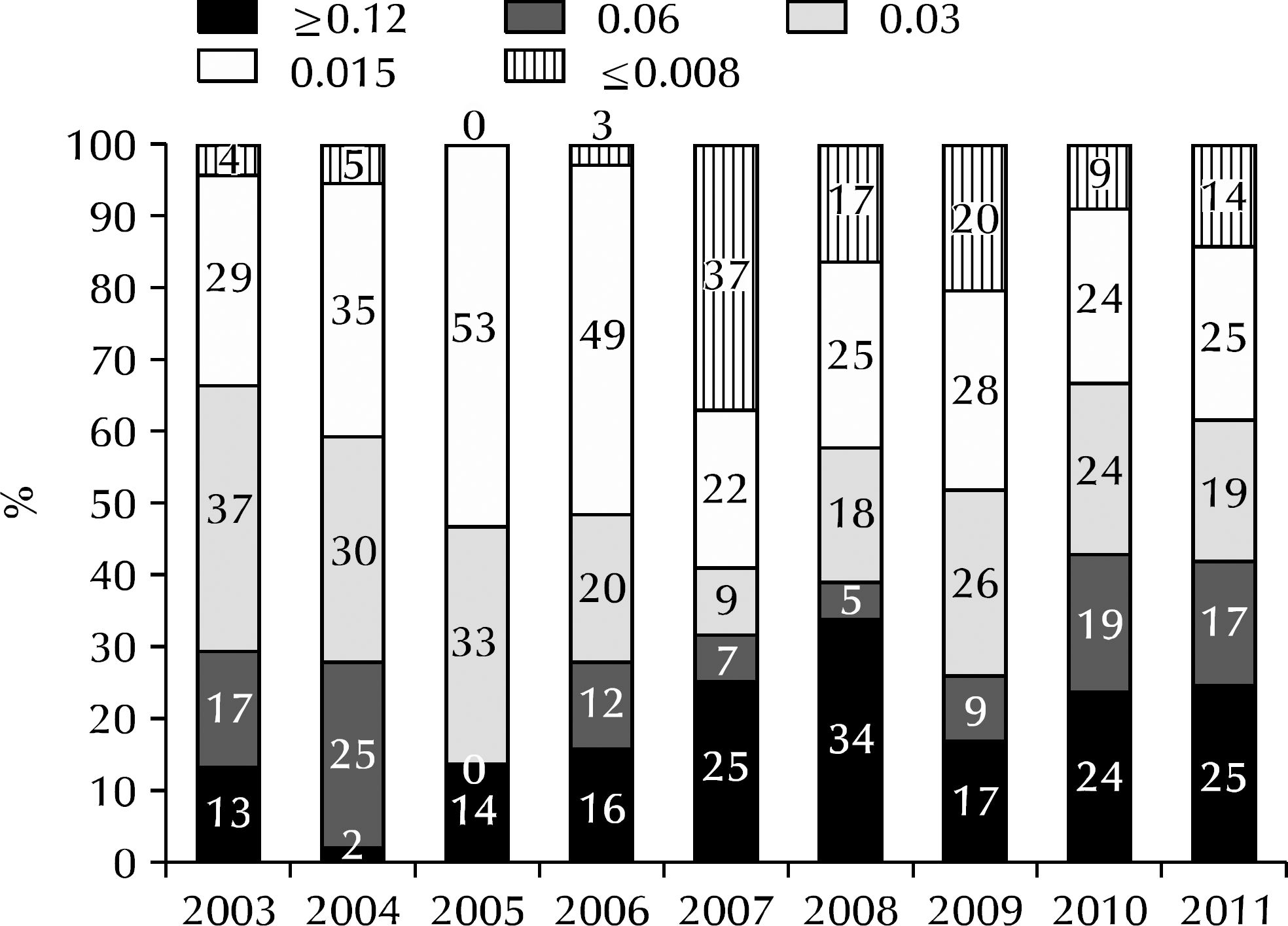
Table 1.
Recent changes of Centers for Disease Control and Prevention in treatment guidelines on gonococcal infection
| Year | Classification | Recommended regimens | Alternative regimens |
|---|---|---|---|
| 200718 | Uncomplicated gonococcal infections of the cervix, urethra, and rectum | Ceftriaxone 125 mg IM in a single dose or cefixime 400 mg PO in a single dose PLUS 1 of the following: Azithromycin 1 g PO in single dosea or doxycycline 100 mg PO twice a day for 7 daysa | Spectinomycin 2 g IM in a single dose or cephalosporin single dose regimens |
| Uncomplicated gonococcal infections of the pharynx | Ceftriaxone 125 mg IM in a single dose PLUS 1 of the following: Azithromycin 1 g PO in single dosea or doxycycline 100 mg PO twice a day for 7 daysa | ||
| 201032 | Uncomplicated gonococcal infections of the cervix, urethra, and rectum | Ceftriaxone 250 mg IM in a single dose or cefixime 400 mg PO in a single dose PLUS 1 of the following: Azithromycin 1 g PO in a single doseb or doxycycline 100 mg twice a day for 7 daysb | Cefpodoxime 400 mg PO Spectinomycin 2 g IM in a single dose |
| Azithromycin 2 g PO in a single doseb | |||
| Uncomplicated gonococcal infections of the pharynx | Ceftriaxone 250 mg IM in a single dose PLUS 1 of the following: | ||
| Azithromycin 1 g PO in a single doseb or doxycycline 100 mg twice a day for 7 daysb | |||
| 20123 | Uncomplicated gonococcal infections of the cervix, urethra, and rectum | Ceftriaxone 250 mg IM in a single dose PLUS 1 of the following: | Cefixime 400 mg PO in a single dose PLUS 1 of the following: |
| Azithromycin 1g PO in a single dose (preferred)b or doxycycline 100 mg twice a day for 7 daysb | Azithromycin 1g PO in a single dose (preferred) or doxycycline 100 mg twice a day for 7 days and test-of-cure in 1 week | ||
| Uncomplicated gonococcal infections of the pharynx | Ceftriaxone 250 mg IM in a single dose PLUS 1 of the following:b | ||
| Azithromycin 1 g PO in a single dose (preferred)b or doxycycline 100 mg twice a day for 7 daysb |
Table 2.
2011 Korean guideline on gonococcal infection
| Classification | Recommended regimens | Alternative regimens |
|---|---|---|
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum | Ceftriaxone 250 mg IM in a single dose or cefixime 400 mg PO in a single dose PLUS 1 of the following: | Spectinomycin 2 g IM in a single dose PLUS 1 of the following: |
| Azithromycin 1 g PO in single dosea or doxycycline 100 mg PO twice a day for 7 daysa | Azithromycin 1 g PO in single dosea or doxycycline 100 mg PO twice a day for 7 daysa | |
| Uncomplicated gonococcal infections of the pharynx | Ceftriaxone 250 mg IM in a single dose PLUS 1 of the following: | |
| Azithromycin 1 g PO in single dosea or doxycycline 100 mg PO twice a day for 7 daysa |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download