Abstract
Mucosal tissues in the gastrointestinal tract are exposed to a significant number of microorganisms, many of which present a danger to the host. In contrast, the urogenital tract is colonized rather infrequently with bacterial organisms and devoid of physical barriers such as a multilayered mucus or ciliated epithelia, thereby necessitating separate host defense mechanisms. Recurrent urinary tract infection (UTI) represents successful microbial host evasion and poses a major health problem. In recent years, considerable advances have been made in our understanding of the mechanisms underlying the immune homeostasis of the urogenital tract. The system of pathogen-recognition receptors, including the Toll-like receptors, is able to sense danger signaling and thus activate the host immune system of the genitourinary tract. Various soluble antimicrobial molecules, including iron-sequestering proteins, defensins, cathelicidin, and Tamm-Horsfall protein, have been more clearly defined. In addition, involvement of signaling mediators such as cyclic adenosine monophosphate or the circulatory hormone vasopressin in the defense of uropathogenic microbes and maintenance of mucosal integrity has been demonstrated. Beyond this, specific receptors that are hijacked by uropathogenic Escherichia coli in order to enable invasion and survival within the urogenital system, paving the way for chronic forms of UTI, have been identified. The majority of these findings offer novel avenues for conduct of basic and translational research for development of effective therapies against the diverse forms of acute and chronic UTI.
Go to : 
REFERENCES
1. Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med. 1993; 329:1328–34.

2. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002; 113(Suppl 1A):5S–13S.

3. Finer G, Landau D. Pathogenesis of urinary tract infections with normal female anatomy. Lancet Infect Dis. 2004; 4:631–5.

4. Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997; 11:551–81.

5. Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am. 1997; 11:513–29.

6. Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000; 97:8829–35.

7. Welch RA, Burland V, Plunkett G 3rd, Redford P, Roesch P, Rasko D, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2002; 99:17020–4.

8. Brumfitt W, Gargan RA, Hamilton-Miller JM. Periurethral enterobacterial carriage preceding urinary infection. Lancet. 1987; 1:824–6.

9. Marrs CF, Zhang L, Foxman B. Escherichia coli mediated urinary tract infections: are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol Lett. 2005; 252:183–90.
10. Roos V, Schembri MA, Ulett GC, Klemm P. Asymptomatic bacteriuria Escherichia coli strain 83972 carries mutations in the foc locus and is unable to express F1C fimbriae. Microbiology. 2006; 152:1799–806.

11. Bower JM, Eto DS, Mulvey MA. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic. 2005; 6:18–31.

12. Jones CH, Pinkner JS, Roth R, Heuser J, Nicholes AV, Abraham SN, et al. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci U S A. 1995; 92:2081–5.

13. Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000; 19:2803–12.

14. Connell I, Agace W, Klemm P, Schembri M, Mărild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A. 1996; 93:9827–32.

15. Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, et al. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997; 389:636–9.

16. Gbarah A, Gahmberg CG, Ofek I, Jacobi U, Sharon N. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect Immun. 1991; 59:4524–30.

17. Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001; 114:4095–103.
18. Rouschop KM, Sylva M, Teske GJ, Hoedemaeker I, Pals ST, Weening JJ, et al. Urothelial CD44 facilitates Escherichia coli infection of the murine urinary tract. J Immunol. 2006; 177:7225–32.
19. Wu XR, Sun TT, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci U S A. 1996; 93:9630–5.

20. Springall T, Sheerin NS, Abe K, Holers VM, Wan H, Sacks SH. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat Med. 2001; 7:801–6.

21. Li K, Feito MJ, Sacks SH, Sheerin NS. CD46 (membrane cofactor protein) acts as a human epithelial cell receptor for internalization of opsonized uropathogenic Escherichia coli. J Immunol. 2006; 177:2543–51.
22. Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007; 3:e100.

23. Lozahic S, Christiansen D, Manié S, Gerlier D, Billard M, Boucheix C, et al. CD46 (membrane cofactor protein) associates with multiple beta1 integrins and tetraspans. Eur J Immunol. 2000; 30:900–7.
24. Hedlund M, Frendéus B, Wachtler C, Hang L, Fischer H, Svanborg C. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol Microbiol. 2001; 39:542–52.

25. Fischer H, Yamamoto M, Akira S, Beutler B, Svanborg C. Mechanism of pathogen-specific TLR4 activation in the mucosa: fimbriae, recognition receptors and adaptor protein selection. Eur J Immunol. 2006; 36:267–77.

26. Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006; 125:943–55.

27. Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001; 69:4572–9.
28. Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001; 166:1148–55.
29. Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr Opin Microbiol. 1999; 2:99–105.

30. Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998; 282:1494–7.

31. Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007; 18:2810–6.
33. Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005; 6:551–7.

34. Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006; 12:636–41.

35. Abrink M, Larsson E, Gobl A, Hellman L. Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney Int. 2000; 57:2004–10.
36. Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002; 10:1033–43.

37. Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004; 432:917–21.

38. Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006; 103:1834–9.

39. Säemann MD, Weichhart T, Hörl WH, Zlabinger GJ. Tamm-Horsfall protein: a multilayered defence molecule against urinary tract infection. Eur J Clin Invest. 2005; 35:227–35.

40. Weichhart T, Zlabinger GJ, Säemann MD. The multiple functions of Tamm-Horsfall protein in human health and disease: a mystery clears up. Wien Klin Wochenschr. 2005; 117:316–22.

41. Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004; 65:791–7.

42. Parkkinen J, Virkola R, Korhonen TK. Identification of factors in human urine that inhibit the binding of Escherichia coli adhesins. Infect Immun. 1988; 56:2623–30.

43. Säemann MD, Weichhart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest. 2005; 115:468–75.

44. Haraoka M, Hang L, Frendéus B, Godaly G, Burdick M, Strieter R, et al. Neutrophil recruitment and resistance to urinary tract infection. J Infect Dis. 1999; 180:1220–9.

45. Hedges S, Agace W, Svensson M, Sjögren AC, Ceska M, Svanborg C. Uroepithelial cells are part of a mucosal cytokine network. Infect Immun. 1994; 62:2315–21.

46. Hang L, Frendéus B, Godaly G, Svanborg C. Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. J Infect Dis. 2000; 182:1738–48.

48. Bäckhed F, Söderhäll M, Ekman P, Normark S, Richter-Dahlfors A. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell Microbiol. 2001; 3:153–8.

49. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801.

51. Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003; 74:479–85.

52. Shahin RD, Engberg I, Hagberg L, Svanborg Edén C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987; 138:3475–80.
53. Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 2003; 100:4203–8.

54. Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, et al. Renal collecting duct epithelial cells react to pye-lonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol. 2006; 177:4773–84.
55. Zager RA, Johnson AC, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol. 2007; 292:F304–12.

56. Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, et al. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol. 2002; 169:2026–33.

57. Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001; 2:346–52.

58. Hung CC, Chang CT, Chen KH, Tian YC, Wu MS, Pan MJ, et al. Upregulation of chemokine CXCL1/KC by leptospiral membrane lipoprotein preparation in renal tubule epithelial cells. Kidney Int. 2006; 69:1814–22.

59. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001; 410:1099–103.

60. Lane MC, Alteri CJ, Smith SN, Mobley HL. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A. 2007; 104:16669–74.

61. Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, et al. Cutting edge: Tlr5-/- mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007; 178:4717–20.
62. Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003; 198:1563–72.

63. Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004; 303:1522–6.

64. Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007; 1:287–98.

65. Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007; 13:625–30.

66. Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008; 14:399–406.

67. Newman RM, Salunkhe P, Godzik A, Reed JC. Identification and characterization of a novel bacterial virulence factor that shares homology with mammalian Toll/interleukin-1 receptor family proteins. Infect Immun. 2006; 74:594–601.

68. Merk K, Borelli C, Korting HC. Lactobacilli - bacteria-host interactions with special regard to the urogenital tract. Int J Med Microbiol. 2005; 295:9–18.

69. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004; 118:229–41.

70. Svanborg C, Bergsten G, Fischer H, Godaly G, Gustafsson M, Karpman D, et al. Uropathogenic Escherichia coli as a model of host-parasite interaction. Curr Opin Microbiol. 2006; 9:33–9.

71. Frendéus B, Wachtler C, Hedlund M, Fischer H, Samuelsson P, Svensson M, et al. Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol Microbiol. 2001; 40:37–51.

72. McNamara N, Gallup M, Sucher A, Maltseva I, McKemy D, Basbaum C. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate Erk1/2. Am J Respir Cell Mol Biol. 2006; 34:653–60.

73. Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003; 4:1247–53.

74. Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004; 6:1361–7.

75. Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun. 2004; 72:3179–86.

76. Schilling JD, Martin SM, Hunstad DA, Patel KP, Mulvey MA, Justice SS, et al. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect Immun. 2003; 71:1470–80.
77. Jones-Carson J, Balish E, Uehling DT. Susceptibility of immunodeficient gene-knockout mice to urinary tract infection. J Urol. 1999; 161:338–41.

78. Hopkins WJ, Uehling DT, Balish E. Local and systemic antibody responses accompany spontaneous resolution of experimental cystitis in cynomolgus monkeys. Infect Immun. 1987; 55:1951–6.

79. Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner JS, Burlein J, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997; 276:607–11.
Go to : 
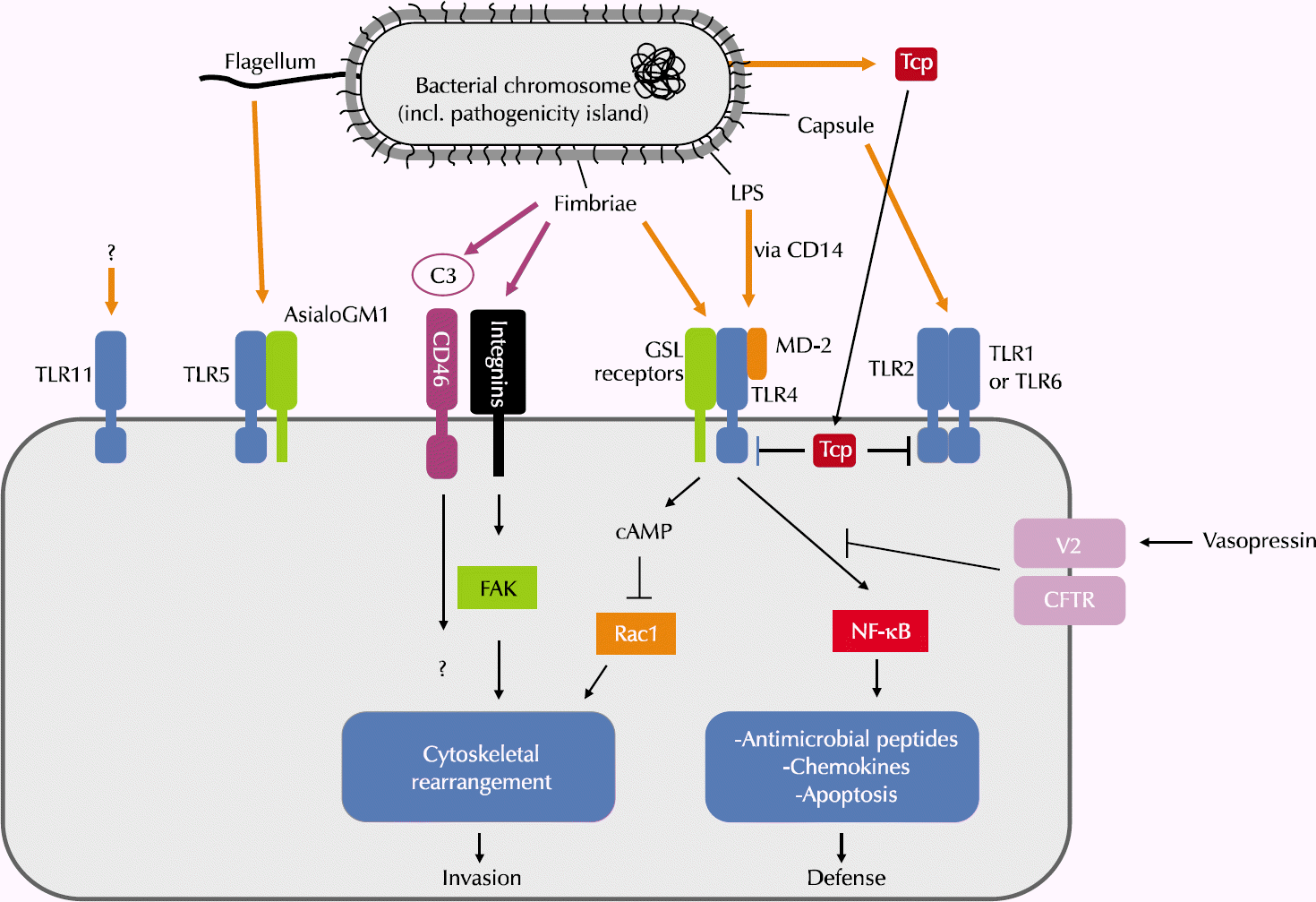 | Fig. 1.The diversity of molecular mechanisms of urinary epithelial cells. LPS: lipopolysaccharide, TLR: toll-like receptor, GSL: glycosphingolipid, Tcp: toll/ interleukin-1 receptor (TIR)-domain-containing protein, FAK: focal adhesion kinase, CFTR: cystic fibrosis transmembrane conductance regulator. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download