This article has been
cited by other articles in ScienceCentral.
Abstract
The effect of human saliva on the flow properties of pudding-like thickened water prepared with commercial food thickeners was investigated, and their viscosity differences were also compared as a function of salivary reaction time (0-60 min after the addition of saliva). Food thickeners used in this study were starch-based (SB), gum-containing starch-based (GSB), and gumbased (GB) commercial thickeners marketed in Korea. GB showed no significant reduction in viscosity upon contact with human saliva during the salivary reaction. In contrast, SB almost completely lost its viscosity shortly after the addition of saliva, and GSB significantly reduced its viscosity after 20 min of reaction time but retained its viscosity. The results of this study indicate that GB can enhance the swallowing safety of dysphagic patients by retaining a stable viscosity level without the reduction of viscosity during consumption of thickened fluids, whereas SB may increase the possibility of aspiration owing to a rapid decrease of viscosity upon contact with human saliva.
Keywords: Food thickener, Salivary reaction time, Flow property, Viscosity
Introduction
Recently, the number of people with dysphagia, which is defined as difficulty swallowing or the inability to swallow solid and/or liquid foods, is increasing in Korea owing to the rapid increase of the aging population [
1]. It is known that people with inadequately managed dysphagia can be at risk of choking, aspiration, aspiration pneumonia, dehydration, weight loss, and malnutrition [
2]. Therefore, commercial food thickeners are very useful in fluid foods for treatment of individuals with dysphagia, with the aim of increasing or modifying the fluid's viscosity to slow the flow rate of fluid transported through the pharynx, thus reducing the risk of aspiration [
3]. However, the correct viscosity is very important for treating dysphagia because fluids with low viscosity may be more likely to enter the airway, resulting in aspiration, and those with high viscosity can be difficult to swallow, resulting in residue within the oropharynx, which is often aspirated [
4].
Commercially available food thickeners for the management of dysphagia have starches or gums as the main active ingredient. However, it is well known that starch is hydrolyzed by the salivary amylase present in saliva, breaking it down into simple carbohydrates [
2]. Therefore, it can be assumed that starch-based thickeners are highly sensitive to amylase in saliva, leading to the decreased viscosity of thickened fluids during consumption. In contrast, it can be expected that fluids thickened with gum-based thickener are less sensitive to thinning by saliva during consumption when compared to those prepared with starch-based thickeners. Even though the flow properties of thickened fluids with thickeners have been extensively studied, no research has been reported to date on the effect of human saliva on the flow properties of thickened fluids prepared with commercial food thickeners, except for Hanson et al. [
5] who found that salivary amylase reduced the viscosity of drinks thickened to a custard-like consistency with starch-based thickeners marketed in the U.K. In the present study, three commercially available food thickeners, which are based on starch, gum-containing starch, and gum, are selected because they are widely used as favorable food thickeners in Korea. In addition, viscosity measurements are performed at a pudding-like consistency because this is a common target viscosity in general practice in Korea. Thus, this study focuses on the effect of human saliva on the viscosity of pudding-like thickened waters prepared with different food thickeners marketed in Korea as a function of salivary reaction time and thickener type.
Materials and Methods
Thickeners and sample preparation
Three commercial food thickeners marketed in Korea were selected: starch-based thickener (SB) (composite of modified corn starch and dextrin), gum-containing starch-based thickener (GSB) (composite of modified tapioca starch, xanthan gum, locust bean gum, and dextrin) and gum-based thickener (GB) (composite of xanthan gum, guar gum, and dextrin). All food thickeners were obtained from their manufacturers (Hormel Health Labs, Savannah, GA, USA; Nisshin OilliO Group Ltd., Tokyo, Japan; Rheosfood Inc., Seoul, Korea, respectively). The thickened waters were prepared by mixing the food thickeners with bottled water (JPDC, Jeju, Korea) at 25 ± 0.1℃ with stirring for 1 min with mild agitation provided by a magnetic stirrer and then stabilized for 1 hr before the viscosity measurement. The amount of thickener used was consistent with clinical practice, which is based on the manufacturers recommendations for producing a pudding-like fluid.
Saliva extraction
Saliva was collected from a healthy male (27 years) each day between 10 a.m. and 11 a.m. prior to a experiment. The volunteer was asked not to eat or drink for 1 hr prior to collection. The volunteer rinsed his mouth with water before saliva collection, then the first 1 mL of saliva was discarded. The total amount of saliva collected was 8 mL over the course of 15-20 min. This saliva was kept in a refrigerator at 5℃ until use. Before use, the collected saliva was first vortex mixed for 20 sec. To prevent further salivary reaction occurring during the measurement of flow properties of thickened samples with added saliva, an acidic solution (10% w/v, pH 1.65) prepared with citric acid powder (Jungbunzlauer Austria AG, Wein, Austria) was used as an amylase activity inhibitor [
2].
Flow properties
Flow properties of a thickened fluid were measured with a Carri-Med CSL
2 100 rheometer (TA Instruments, New Castle, DE, USA), using a parallel plate system (4 cm diameter) at a gap of 500 µm. The temperature of a sample during measurement was maintained at 25℃. Steady shear viscosity data were obtained from a power law model (Eq. 1) over the shear rate range of 0.1-100 s
-1.
In this equation, σ (Pa) is the shear stress,
·γ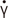
(s
-1) is the shear rate, K (Pa·s
n) is the consistency index, and n is the flow behavior index. The apparent viscosity (η
a,50) at 50 s
-1, a reference shear rate for swallowing, was calculated from the K and n values. Human saliva (1 mL) was added to the thickened waters (10 mL) prepared with different food thickeners and then stirred for 20 sec, and enzyme activity inhibitor (1 mL) was added at different salivary reaction times (0-60 min) after adding saliva. A thickened sample with no added saliva (control) was also prepared to compare with samples with saliva added. These thickened water samples were immediately transferred to the rheometer plate at 25℃ to measure their viscosity. All samples were allowed to rest at 25℃ for 5 min in order to relax the samples before the viscosity measurements.
Statistical analysis
All results are expressed as mean ± standard deviation. Analysis of variance was performed using Statistical Analysis System software (version 9.2, SAS Institute, Cary, NC, USA). Differences in means were determined using Duncan's multiple-range test.
Results
The shear stress (σ) versus shear rate (
·γ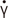
) data for thickened water samples added with saliva at the control (no added saliva) and 60 min after addition of saliva at 25℃ are shown in
Figure 1. The experimental results of σ and
·γ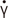
of all samples, except for SB with saliva, were well fitted to the simple power law model (Eq. 1) with high determination coefficients (R
2 = 0.96-0.99) (data not given). In this study, the flow curves of SB with saliva could not be obtained because of the formation of an unstable structure of SB owing to the addition of saliva. Therefore, for SB samples with added saliva, we measured the viscosity (η
a,50) only at a shear rate of 50 s
-1 without the application of the power law model. Thickened samples with different thickeners also exhibited a clear trend of shear-thinning behavior. In particular, the GSB and GB samples exhibited higher shear-thinning behavior when compared to SB. There was a difference in flow curves of GSB between the control and the GSB sample with added saliva at a reaction time of 60 min (
Figure 1). In addition, GSB and GB food thickeners also produced different flow properties at a 60 min of salivary reaction time after the contact with saliva.
Table 1 shows the apparent viscosity (η
a,50) of thickened waters with three different thickeners (SB, GSB, and GB) as a function of salivary reaction time (0-60 min) after the addition of saliva. The reduction in viscosity for SB and GSB on saliva addition was significant at 0 min and 20 min of reaction time, respectively.
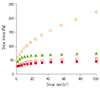
Figure 2 also shows the % changes in initial viscosity (control) values based on the control samples. For SB, the viscosity values greatly reduced after the contact with saliva irrespective of the reaction time, showing the greatest reduction of 99.9% of initial viscosity and no difference in η
a,50 values between salivary reaction times of 0 and 60 min, while for GSB, the viscosity values significantly reduced after 20 min of reaction time and then retained stable (12.2-12.7% of initial viscosity). In contrast, there was no significant difference in the viscosity values of GB between the control and the sample mixed with saliva. These results were confirmed from the photographs of the viscosity differences between the control and the thickened water with added saliva, as shown in
Figure 3.
Discussion
Thickened fluids prepared with commercial food thickeners are commonly used for consumption by used for dysphagic patients patients, who take a long time to eat or drink owing to swallowing difficulties. Therefore, from a clinical perspective, it is very important to observe how food thickeners perform with thickened fluids when mixed with human saliva over an appropriate salivary reaction time because the thinning of thickened fluids after contact with saliva has been reported [
5].
Figure 1 shows that the GB and GSB with no added saliva showed higher shear-thinning flow behaviors and lower viscosity values compared to those for SB, possibly owing to the presence of xanthan in the food thickener [
6]. Urlacher and Noble [
7] also reported that such high shear-thinning behavior may be owing to the unique rigid, rod-like conformation and high molecular weight of xanthan gum. These observed results followed similar trends to those previously found in thickened fluids prepared with various xanthan gum-based thickeners [
8]. A noticeable difference was observed in viscosity values between food thickeners as a function of salivary reaction time (0-60 min), as shown in
Table 1 and
Figure 2.
Figure 3 also shows the photographs of the viscosity differences between the control and thickened water with added saliva. In comparison of η
a,50 values of the SG and GSB samples, the lower viscosity values of the samples mixed with saliva can be explained by the hydrolysis of the starch component contained in SB and GSB due to the salivary amylase present in saliva [
9]. Among food thickeners, GB was not influenced by salivary amylase owing to the inactivation of amylase with gums, showing that GB is the most suitable food thickener for dysphagic patients. These results suggest that the addition of saliva had a more pronounced effect on the viscosity of thickened fluids prepared with food thickeners (SB and GSB) containing starch. Similar observations were reported by Hanson et al. [
5] for food thickeners composed of corn starch alone or corn starch with the addition of gums. From these results, it was shown that dysphagic patients cannot receive thickened fluids with the correct viscosity for the swallowing safety because thickened fluids with food thickeners containing starch are greatly influenced by the type of thickeners in the presence of saliva, suggesting the need for the preparation of thickened fluids with a stable viscosity after contact with saliva in mouth. Therefore, it can be concluded that GB is a better food thickener for the treatment of dysphagic patients because the GB thickened fluids are not influenced by saliva during the consumption of fluid foods.
Conclusion
Changes in the viscosity of thickened waters over salivary reaction time are more pronounced in a food thickener containing starch alone owing to the complete breakdown of starch caused by the strong activation of salivary amylase. Therefore, it is desirable to use food thickeners containing gums for the treatment of patients with dysphagia because gums in food thickeners are not susceptible to digestion by amylase and provide a stable viscosity. These results will be useful for developing new food thickeners or thickening products with a stable viscosity in the presence of human saliva. However, more investigations need to be carried because the viscosity of thickened fluids with saliva can be greatly influenced by the amount of salivary amylase. In terms of clinical practice in patients with dysphagia, an additional study is also needed on various thickened fluids with saliva obtained from dysphagic patients to extend the results of this study.
Figures and Tables
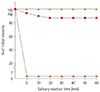 | Figure 2Effect of saliva addition on the viscosity reduction of thickened waters at different salivary reaction times (0, 10, 20, 30, 40, 50, and 60 min) at 25℃: (●) SB: starch-based, (■) GSB: gum-containing starch-based, (▲) GB: gum-based. 100% of initial viscosity means the viscosity values of the control samples (no added saliva) with different food thickeners (SB, SGB, and GB).
|
 | Figure 3Pudding-like thickened water samples prepared with different food thickeners (SB, GSB, and GB). Control: thickened water with no added saliva, Sample with saliva: thickened water with added saliva at 60 min of salivary reaction time, SB: starchbased, GSB: gum-containing starch-based, GB: gum-based.
|
Table 1
Effect of salivary reaction time on apparent viscosity (ηa,50) values of thickened waters prepared with different food
thickeners

Salivary reaction time
(min) |
Apparent viscosity (ηa,50, Pa) |
|
SB |
GSB |
GB |
|
Control |
3.29 ± 0.03a
|
1.01 ± 0.02a
|
1.33 ± 0.01a
|
|
0 |
0.01 ± 0.01b
|
0.95 ± 0.00b
|
1.33 ± 0.01a
|
|
10 |
0.01 ± 0.01b
|
0.92 ± 0.00c
|
1.33 ± 0.01a
|
|
20 |
0.01 ± 0.00b
|
0.88 ± 0.01d
|
1.33 ± 0.00a
|
|
30 |
0.01 ± 0.00b
|
0.88 ± 0.02d
|
1.32 ± 0.02a
|
|
40 |
0.01 ± 0.00b
|
0.88 ± 0.02d
|
1.33 ± 0.01a
|
|
50 |
0.01 ± 0.00b
|
0.88 ± 0.02d
|
1.33 ± 0.03a
|
|
60 |
0.01 ± 0.00b
|
0.88 ± 0.01d
|
1.32 ± 0.01a
|

Acknowlegement
We thank Rheosfood Inc. for providing the free gum-based food thickener (Visco-up) sample.
References
1. Kim SG, Yoo B. Viscosity of dysphagia-oriented cold-thickened beverages: effect of setting time at refrigeration temperature. Int J Lang Commun Disord. 2015; 50:397–402.

2. Hanson B, Cox B, Kaliviotis E, Smith CH. Effects of saliva on starch-thickened drinks with acidic and neutral pH. Dysphagia. 2012; 27:427–435.

3. Garcia JM, Chambers E 4th, Matta Z, Clark M. Viscosity measurements of nectar- and honey-thick liquids: product, liquid, and time comparisons. Dysphagia. 2005; 20:325–335.

4. Glassburn DL, Deem JF. Thickener viscosity in dysphagia management: variability among speech-language pathologists. Dysphagia. 1998; 13:218–222.

5. Hanson B, O'Leary MT, Smith CH. The effect of saliva on the viscosity of thickened drinks. Dysphagia. 2012; 27:10–9.

6. Cho HM, Yoo B. Rheological characteristics of cold thickened beverages containing xanthan gum-based food thickeners used for dysphagia diets. J Acad Nutr Diet. 2015; 115:106–111.

7. Urlacher B, Noble O. Xanthan gum. In : Imeson AP, editor. Thickening and gelling agents for food. New York (NY): Chapman & Hall;1997. p. 284–311.
8. Seo CW, Yoo B. Steady and dynamic shear rheological properties of gum-based food thickeners used for diet modification of patients with dysphagia: effect of concentration. Dysphagia. 2013; 28:205–211.

9. Nantanga KK, Chan E, Suleman S, Bertoft E, Seetharaman K. Differences in structures of starch hydrolysates using saliva from different individuals. Starke. 2013; 65:709–713.

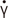 (s-1) is the shear rate, K (Pa·sn) is the consistency index, and n is the flow behavior index. The apparent viscosity (ηa,50) at 50 s-1, a reference shear rate for swallowing, was calculated from the K and n values. Human saliva (1 mL) was added to the thickened waters (10 mL) prepared with different food thickeners and then stirred for 20 sec, and enzyme activity inhibitor (1 mL) was added at different salivary reaction times (0-60 min) after adding saliva. A thickened sample with no added saliva (control) was also prepared to compare with samples with saliva added. These thickened water samples were immediately transferred to the rheometer plate at 25℃ to measure their viscosity. All samples were allowed to rest at 25℃ for 5 min in order to relax the samples before the viscosity measurements.
(s-1) is the shear rate, K (Pa·sn) is the consistency index, and n is the flow behavior index. The apparent viscosity (ηa,50) at 50 s-1, a reference shear rate for swallowing, was calculated from the K and n values. Human saliva (1 mL) was added to the thickened waters (10 mL) prepared with different food thickeners and then stirred for 20 sec, and enzyme activity inhibitor (1 mL) was added at different salivary reaction times (0-60 min) after adding saliva. A thickened sample with no added saliva (control) was also prepared to compare with samples with saliva added. These thickened water samples were immediately transferred to the rheometer plate at 25℃ to measure their viscosity. All samples were allowed to rest at 25℃ for 5 min in order to relax the samples before the viscosity measurements.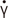 ) data for thickened water samples added with saliva at the control (no added saliva) and 60 min after addition of saliva at 25℃ are shown in Figure 1. The experimental results of σ and ·γ
) data for thickened water samples added with saliva at the control (no added saliva) and 60 min after addition of saliva at 25℃ are shown in Figure 1. The experimental results of σ and ·γ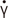 of all samples, except for SB with saliva, were well fitted to the simple power law model (Eq. 1) with high determination coefficients (R2 = 0.96-0.99) (data not given). In this study, the flow curves of SB with saliva could not be obtained because of the formation of an unstable structure of SB owing to the addition of saliva. Therefore, for SB samples with added saliva, we measured the viscosity (ηa,50) only at a shear rate of 50 s-1 without the application of the power law model. Thickened samples with different thickeners also exhibited a clear trend of shear-thinning behavior. In particular, the GSB and GB samples exhibited higher shear-thinning behavior when compared to SB. There was a difference in flow curves of GSB between the control and the GSB sample with added saliva at a reaction time of 60 min (Figure 1). In addition, GSB and GB food thickeners also produced different flow properties at a 60 min of salivary reaction time after the contact with saliva.
of all samples, except for SB with saliva, were well fitted to the simple power law model (Eq. 1) with high determination coefficients (R2 = 0.96-0.99) (data not given). In this study, the flow curves of SB with saliva could not be obtained because of the formation of an unstable structure of SB owing to the addition of saliva. Therefore, for SB samples with added saliva, we measured the viscosity (ηa,50) only at a shear rate of 50 s-1 without the application of the power law model. Thickened samples with different thickeners also exhibited a clear trend of shear-thinning behavior. In particular, the GSB and GB samples exhibited higher shear-thinning behavior when compared to SB. There was a difference in flow curves of GSB between the control and the GSB sample with added saliva at a reaction time of 60 min (Figure 1). In addition, GSB and GB food thickeners also produced different flow properties at a 60 min of salivary reaction time after the contact with saliva.



 PDF
PDF ePub
ePub Citation
Citation Print
Print











 XML Download
XML Download